Hydrogen disulfide
Hydrogen disulfide is the inorganic compound with the formula H2S2. This hydrogen chalcogenide is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide (H2S) and elemental sulfur.[1]
 | |
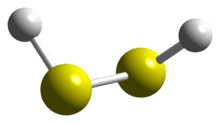 | |
| Names | |
|---|---|
| IUPAC name
Dihydrogen disulfide | |
| Other names
Hydrogen disulphide; Hydrogen persulfide; Hydrogen persulphide; Thiosulfenic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H2S2 | |
| Molar mass | 66.14 g·mol−1 |
| Appearance | yellow liquid |
| Density | 1.334 g cm−3 |
| Melting point | −89.6 °C (−129.3 °F; 183.6 K) |
| Boiling point | 70.7 °C (159.3 °F; 343.8 K) |
| Conjugate base | Disulfanide HS− 2 |
| Hazards | |
| Flash point | flammable |
| Related compounds | |
Related compounds |
Hydrogen sulfide Hydrogen peroxide Hydrogen diselenide Hydrogen ditelluride Disulfur dichloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
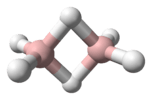
Structure
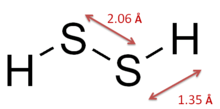
The structure of hydrogen disulfide is similar to that of hydrogen peroxide, with C2 point group symmetry. Both molecules are distinctly nonplanar. The dihedral angle is 90.6°, compared with 111.5° in H2O2. The H−S−S bond angle is 92°, close to 90º for unhybridized divalent sulfur.[1]
Synthesis and reactions
Hydrogen disulfide can be synthesised by dissolving alkali metal or alkaline earth metal polysulfides in water. When the solution is mixed with concentrated hydrochloric acid at −15 °C, a yellow oil consisting a mixture of polysulfanes (H2Sn) will pool below the aqueous layer. Fractional distillation of this oil gives hydrogen disulfide separate from any other polysulfides (mostly trisulfide).[2][3][4][1]
Hydrogen disulfide readily decomposes under ambient conditions to hydrogen sulfide and sulfur.[3] In organosulfur chemistry, hydrogen disulfide adds to alkenes to give disulfides and thiols.[5]
Quantum tunneling and its suppression in deuterium disulfide
The deuterated form of hydrogen disulfide DSSD, has a similar geometry to HSSH, but its tunneling time is slower, making it a convenient test case for the quantum Zeno effect, in which frequent observation of a quantum system suppresses its normal evolution. Trost and Hornberger[6] have calculated that while an isolated DSSD molecule would spontaneously oscillate between left and right chiral forms with a period of 5.6 milliseconds, the presence of a small amount of inert helium gas should stabilize the chiral states, the collisions of the helium atoms in effect "observing" the molecule's momentary chirality and so suppressing spontaneous evolution to the other chiral state.[7]
Health effects
Hydrogen disulfide has been described as "having a severe and irritating odour" that is similar to camphor or SCl
2, causing "tears and a smarting sensation in the nostrils".[3] If it is present in high concentrations, it can cause dizziness, disorientation and ultimately unconsciousness.[8]
References
- R. Steudel "Inorganic Polysulfanes H2Sn with n > 1" in Elemental Sulfur and Sulfur-Rich Compounds II (Topics in Current Chemistry) 2003, Volume 231, pp 99–125. doi:10.1007/b13182
- De, A. K. (2001-01-15). A Text Book of Inorganic Chemistry. ISBN 978-81-224-1384-7.
- Walton and Parson; Parsons, Llewellyn B. (1921). "Preparation and Properties of the Persulfides of Hydrogen". J. Am. Chem. Soc. 43 (12): 2539–48. doi:10.1021/ja01445a008.
- Georg Brauer: Handbook of Preparative Inorganic Chemistry Volume I, page 391, Wiley, 1963.
- Hazardous Reagents, Robinson Brothers
- Trost, J.; Hornberger, K. (2009). "Hund's Paradox and the Collisional Stabilization of Chiral Molecules". Phys. Rev. Lett. 103 (2): 023202. arXiv:0811.2140. Bibcode:2009PhRvL.103b3202T. doi:10.1103/PhysRevLett.103.023202. PMID 19659202.
- Month-long calculation solves 82-year-old quantum paradox, Physics Today, September 2009, p. 16
- Stein, Wilkinson, G (2007). Seminars in general adult psychiatry. Royal College of Psychiatrists. ISBN 978-1-904671-44-2.