AB-005
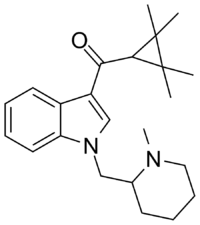
AB-005 or [1-[(1-methylpiperidin-2-yl)methyl]-1H-indol-3-yl](2,2,3,3-tetramethylcyclopropyl)-methanone is a designer drug offered by online vendors as a cannabimimetic agent. The structure and pharmacological activity of AB-005 was published in 2010, prior to its commercial availability in 2012, where it was reported to have high affinity for both CB1 (Ki = 5.5 nM) and CB2 receptors (Ki = 0.48 nM).[1] AB-005 features groups found in other previously reported synthetic cannabinoids: the tetramethylcyclopropane group of UR-144 and XLR-11 as well as the (1-methyl-2-piperidinyl)methyl substituent of AM-1248 and AM-1220. No information regarding the in vivo activity of AB-005 has been published, and only anecdotal reports are known of its psychoactivity in humans.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H32N2O |
| Molar mass | 352.522 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Legal status
In 2013, psychoactive products in New Zealand containing this drug were given interim approval under psychoactive substances legislation.[2]
References
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
- "Interim Product Approvals". Ministry of Health. Archived from the original on 21 August 2013. Retrieved 2 January 2013.