Ungulate
Ungulates (pronounced /ˈʌŋɡjəleɪts/ UNG-gyə-layts) are members of a diverse clade of primarily large mammals with hooves. These include odd-toed ungulates such as horses, rhinoceroses and tapirs, and even-toed ungulates such as cattle, pigs, giraffes, camels, sheep, deer, and hippopotamuses. Cetaceans are also even-toed ungulates although they do not have hooves. Most terrestrial ungulates use the tips of their toes, usually hoofed body weight while moving.
| Ungulate | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Clade: | Ferungulata |
| Clade: | Ungulata Linnaeus, 1766 |
| Orders and Clades | |
| |
The term means, roughly, "being hoofed" or "hoofed animal". As a descriptive term, "ungulate" normally excludes cetaceans (whales, dolphins, porpoises), as they do not possess most of the typical morphological characteristics of ungulates, but recent discoveries indicate that they were descended from early artiodactyls.[4] Ungulates are typically herbivorous and many employ specialized gut-bacteria to allow them to digest cellulose. Some modern species, such as pigs, are omnivorous, while some prehistoric species, such as mesonychians, were carnivorous.
Classifications
History
Ungulata is a clade (or in some taxonomies, a grand order) of mammals. The two orders of ungulates were the Perissodactyla (odd-toed ungulates) and Artiodactyla (even-toed ungulates). Hyracoidea (hyraxes), Sirenia (sea cows) (dugongs and manatees) and Proboscidea (elephants) were in the past included in a superorder called Paenungulata which was grouped with the ungulata. These three orders were now considered a clade and grouped in the Afrotheria clade while Ungulata is now grouped under the Laurasiatheria clade.
In 2009 morphological[5][6][7][8] and molecular[9][10] work found that aardvarks, hyraxes, sea cows, and elephants were more closely related to each other and to sengis, tenrecs, and golden moles than to the perissodactyls and artiodactyls, and form the clade Afrotheria. Elephants, sea cows, and hyraxes were grouped together in the clade Paenungulata, while the aardvark has been considered as either a close relative to them or a close relative to sengis in the clade Afroinsectiphilia.[11] This is a striking example of convergent evolution.[12]
There is now some dispute as to whether this smaller Ungulata is a cladistic (evolution-based) group, or merely a phenetic group (form taxon) or folk taxon (similar, but not necessarily related). Some studies have indeed found the mesaxonian ungulates and paraxonian ungulates to form a monophyletic lineage,[13][14][15] closely related to either the Ferae (the carnivorans and the pangolins)[16][17] in the clade Fereuungulata or to the bats.[18] Other studies found the two orders not that closely related, as some place the perissodactyls as close relatives to bats and Ferae in Pegasoferae[19] and others place the artiodactyls as close relatives to bats.[20]
Taxonomy
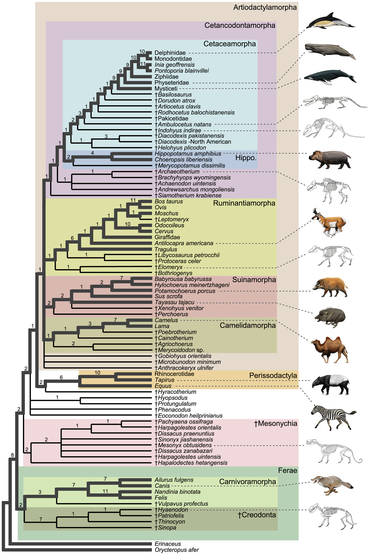
Below is a simplified taxonomy (assuming that ungulates do indeed form a natural grouping) with the extant families, in order of the relationships. Keep in mind that there were still some grey areas of conflict, such as the case with relationship of the pecoran families and the baleen whale families. See each family for the relationships of the species as well as the controversies in their respective article.
- Ungulata (= Euungulata[21])
- Perissodactyla (Mesaxonian ungulates)
- Hippomorpha
- Equidae: Horses, asses and zebras
- Ceratomorpha
- Tapiridae: Tapirs
- Rhinocerotidae: Rhinoceroses
- Hippomorpha
- Artiodactyla (= Cetartiodactyla) (Paraxonian ungulates)
- Tylopoda
- Camelidae: Camels and Llamas
- Artiofabula
- Suina
- Tayassuidae: Peccaries
- Suidae: Pigs
- Cetruminantia
- Ruminantia
- Tragulidae: Chevrotains
- Cervoidea
- Antilocapridae: Pronghorn
- Giraffidae: Giraffes and okapi
- Cervidae: Deer
- Moschidae: Musk deer
- Bovidae: Oxen and antelopes
- Whippomorpha
- Hippopotamidae: Hippopotamuses
- Cetacea
- Mysticeti
- Balaenidae: Bowhead and right whales
- Cetotheriidae: Pygmy right whale
- Eschrichtiidae: Gray Whale
- Balaenopteridae: Rorquals
- Odontoceti
- Physeteroidea
- Physeteridae: Sperm whale
- Kogiidae: Lesser sperm whales
- Platanistoidea
- Platanistidae: Indian river dolphins
- Ziphioidea
- Ziphiidae: Beaked whales
- Lipotoidea
- Lipotidae: Baiji (functionally extinct)
- Inioidea
- Iniidae: Amazonian river dolphins
- Pontoporiidae: La Plata dolphin
- Delphinoidea
- Monodontidae: Beluga and narwhal
- Phocoenidae: Porpoises
- Delphinidae: Oceanic dolphins
- Physeteroidea
- Mysticeti
- Ruminantia
- Suina
- Tylopoda
- Perissodactyla (Mesaxonian ungulates)
Phylogeny
Below is the general consensus of the phylogeny of the ungulate families.[22][23]
| Ungulata |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Evolution

Perissodactyla and Artiodactyla include the majority of large land mammals. These two groups first appeared during the late Paleocene, rapidly spreading to a wide variety of species on numerous continents, and have developed in parallel since that time. Some scientists believed that modern ungulates were descended from an evolutionary grade of mammals known as the condylarths;[24] the earliest known member of the group was the tiny Protungulatum,[25] an ungulate that co-existed with the last of non-avian dinosaurs 66 million years ago; however, many authorities do not consider it a true placental, let alone an ungulate.[26] The enigmatic dinoceratans were among the first large herbivorous mammals, although their exact relationship with other mammals is still debated with one of the theories being that they might just be distant relatives to living ungulates; the most recent study recovers them as within the true ungulate assemblage, closest to Carodnia.[3]
In Australia, the marsupial Chaeropus also developed hooves similar to those of artiodactyls,[27] an example of convergent evolution.
Perissodactyl evolution

Perissodactyls were said to have evolved from the Phenacodontidae, small, sheep-sized animals that were already showing signs of anatomical features that their descendants would inherit (the reduction of digit I and V for example).[29] By the start of the Eocene, 55 million years ago (Mya), they had diversified and spread out to occupy several continents. Horses and tapirs both evolved in North America;[30] rhinoceroses appear to have developed in Asia from tapir-like animals and then colonised the Americas during the middle Eocene (about 45 Mya). Of the approximately 15 families, only three survive (McKenna and Bell, 1997; Hooker, 2005). These families were very diverse in form and size; they included the enormous brontotheres and the bizarre chalicotheres. The largest perissodactyl, an Asian rhinoceros called Paraceratherium, reached 15 tonnes (17 tons), more than twice the weight of an elephant.[31]
It has been found in a cladistic study that the anthracobunids and the desmostylians - two lineages that have been previously classified as Afrotherians (more specifically closer to elephants) - have been classified as a clade that is closely related to the perissodactyls.[1] The desmostylians were large amphibious quadrupeds with massive limbs and a short tail.[32] They grew to 1.8 metres (6 ft) in length and were thought to have weighed more than 200 kilograms (440 lb). Their fossils were known from the northern Pacific Rim,[33] from southern Japan through Russia, the Aleutian Islands and the Pacific coast of North America to the southern tip of Baja California. Their dental and skeletal form suggests desmostylians were aquatic herbivores dependent on littoral habitats. Their name refers to their highly distinctive molars, in which each cusp was modified into hollow columns, so that a typical molar would have resembled a cluster of pipes, or in the case of worn molars, volcanoes. They were the only marine mammals to have gone extinct.
The South American meridiungulates contain the somewhat tapir-like pyrotheres and astrapotheres, the mesaxonic litopterns and the diverse notoungulates. As a whole, meridiungulates were said to have evolved from animals like Hyopsodus.[34] For a while their relationships with other ungulates were a mystery. Some paleontologists have even challenged the monophyly of Meridiungulata by suggesting that the pyrotheres may be more closely related to other mammals, such as Embrithopoda (an African order that were related to elephants) than to other South American ungulates.[35] A recent study based on bone collagen has found that at least litopterns and the notoungulates were closely related to the perissodactyls.[2]
The oldest known fossils assigned to Equidae date from the early Eocene, 54 million years ago. They had been assigned to the genus Hyracotherium, but the type species of that genus is now considered not a member of this family, but the other species have been split off into different genera. These early Equidae were fox-sized animals with three toes on the hind feet, and four on the front feet. They were herbivorous browsers on relatively soft plants, and already adapted for running. The complexity of their brains suggest that they already were alert and intelligent animals.[36] Later species reduced the number of toes, and developed teeth more suited for grinding up grasses and other tough plant food.
Rhinocerotoids diverged from other perissodactyls by the early Eocene. Fossils of Hyrachyus eximus found in North America date to this period. This small hornless ancestor resembled a tapir or small horse more than a rhino. Three families, sometimes grouped together as the superfamily Rhinocerotoidea, evolved in the late Eocene: Hyracodontidae, Amynodontidae and Rhinocerotidae, thus creating an explosion of diversity unmatched for a while until environmental changes drastically eliminated several species.
The first tapirids, such as Heptodon, appeared in the early Eocene.[37] They appeared very similar to modern forms, but were about half the size, and lacked the proboscis. The first true tapirs appeared in the Oligocene. By the Miocene, such genera as Miotapirus were almost indistinguishable from the extant species. Asian and American tapirs were believed to have diverged around 20 to 30 million years ago; and tapirs migrated from North America to South America around 3 million years ago, as part of the Great American Interchange.[38]
Perissodactyls were the dominant group of large terrestrial browsers right through the Oligocene. However, the rise of grasses in the Miocene (about 20 Mya) saw a major change: the artiodactyl species with their more complex stomachs were better able to adapt to a coarse, low-nutrition diet, and soon rose to prominence. Nevertheless, many perissodactyl species survived and prospered until the late Pleistocene (about 10,000 years ago) when they faced the pressure of human hunting and habitat change.
Artiodactyl evolution

The artiodactyls were thought to have evolved from a small group of condylarths, Arctocyonidae, which were unspecialized, superficially raccoon-like to bear-like omnivores from the Early Paleocene (about 65 to 60 million years ago). They had relatively short limbs lacking specializations associated with their relatives (e.g. reduced side digits, fused bones, and hooves),[39] and long, heavy tails. Their primitive anatomy makes it unlikely that they were able to run down prey, but with their powerful proportions, claws, and long canines, they may have been able to overpower smaller animals in surprise attacks.[40] Evidently these mammals soon evolved into two separate lineages: the mesonychians and the artiodactyls.
Mesonychians were depicted as "wolves on hooves" and were the first major mammalian predators, appearing in the Paleocene.[41] Early mesonychids had five digits on their feet, which probably rested flat on the ground during walking (plantigrade locomotion), but later mesonychids had four digits that ended in tiny hooves on all of their toes and were increasingly well adapted to running. Like running members of the even-toed ungulates, mesonychids (Pachyaena, for example) walked on their digits (digitigrade locomotion).[41] Mesonychians fared very poorly at the close of the Eocene epoch, with only one genus, Mongolestes,[42] surviving into the Early Oligocene epoch, as the climate changed and fierce competition arose from the better adapted creodonts.
The first artiodactyls looked like today's chevrotains or pigs: small, short-legged creatures that ate leaves and the soft parts of plants. By the Late Eocene (46 million years ago), the three modern suborders had already developed: Suina (the pig group); Tylopoda (the camel group); and Ruminantia (the goat and cattle group). Nevertheless, artiodactyls were far from dominant at that time: the perissodactyls were much more successful and far more numerous. Artiodactyls survived in niche roles, usually occupying marginal habitats, and it is presumably at that time that they developed their complex digestive systems, which allowed them to survive on lower-grade food. While most artiodactyls were taking over the niches left behind by several extinct perissodactyls, one lineage of artiodactyls began to venture out into the seas.
Cetacean evolution

The traditional theory of cetacean evolution was that cetaceans were related to the mesonychids. These animals had unusual triangular teeth very similar to those of primitive cetaceans. This is why scientists long believed that cetaceans evolved from a form of mesonychid. Today many scientists believe cetaceans evolved from the same stock that gave rise to hippopotamuses. This hypothesized ancestral group likely split into two branches around 54 million years ago.[4] One branch would evolve into cetaceans, possibly beginning about 52 million years ago with the proto-whale Pakicetus and other early cetacean ancestors collectively known as Archaeoceti, which eventually underwent aquatic adaptation into the completely aquatic cetaceans.[43] The other branch became the anthracotheres, a large family of four-legged beasts, the earliest of whom in the late Eocene would have resembled skinny hippopotamuses with comparatively small and narrow heads. All branches of the anthracotheres, except that which evolved into Hippopotamidae, became extinct during the Pliocene without leaving any descendants.[44] The family Raoellidae is said to be the closest artiodactyl family to the cetaceans.[45][46] Consequentially, new theories in cetacean evolution hypothesize that whales and their ancestors escaped predation, not competition, by slowly adapting to the ocean.[47][48][49]
Characteristics

Ungulates were in high diversity in response to sexual selection and ecological events; the majority of ungulates lack a collar bone.[50] Terrestrial ungulates were for the most part herbivores, with some of them being grazers. However, there were exceptions to this as pigs, peccaries, hippos and duikers were known to have an omnivorous diet. Some cetaceans were the only modern ungulates that were carnivores; baleen whales consume significantly smaller animals in relation to their body size, such as small species of fish and krill; toothed whales, depending on the species, can consume a wide range of species: squid, fish, sharks, and other species of mammals such as seals and other whales. In terms of ecosystem ungulates have colonized all corners of the planet, from mountains to the ocean depths; grasslands to deserts and some have been domesticated by humans.
Anatomy
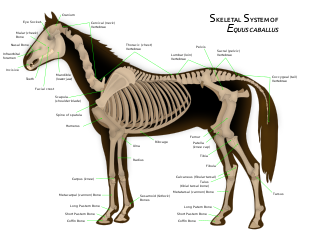
Ungulates have developed specialized adaptations, especially in the areas of cranial appendages, dentition, and leg morphology including the modification of the astragalus (one of the ankle bones at the end of the lower leg) with a short, robust head.
Hooves

The hoof is the tip of a toe of an ungulate mammal, strengthened by a thick horny (keratin) covering. The hoof consists of a hard or rubbery sole, and a hard wall formed by a thick nail rolled around the tip of the toe. The weight of the animal is normally borne by both the sole and the edge of the hoof wall. Hooves grow continuously, and were constantly worn down by use. In most modern ungulates, the radius and ulna were fused along the length of the forelimb; early ungulates, such as the arctocyonids, did not share this unique skeletal structure.[51] The fusion of the radius and ulna prevents an ungulate from rotating its forelimb. Since this skeletal structure has no specific function in ungulates, it is considered a homologous characteristic that ungulates share with other mammals. This trait would have been passed down from a common ancestor. While the two orders of ungulates colloquial names were based on the number of toes of their members ("odd-toed" for the perissodactyls and "even-toed" for the terrestrial artiodactyls), it is not an accurate reason they were grouped. Tapirs have four toes in the front, yet they were members of the "odd-toed" order; peccaries and modern cetaceans were members of the "even-toed" order, yet peccaries have three toes in the front and whales were an extreme example as they have flippers instead of hooves. Scientists had classified them according to the distribution of their weight to their toes.
Perissodactyls have a mesaxonic foot meaning that the weight is distributed on the third toe on all legs thanks to the plane symmetry of their feet. There has been reduction of toes from the common ancestor, with the classic example being horses with their single hooves. In consequence, there was an alternative name for the perissodactyls the nearly obsolete Mesaxonia. Perissodactyls were not the only lineage of mammals to have evolved this trait; the meridiungulates have evolved mesaxonic feet numerous times.
Terrestrial artiodactyls have a paraxonic foot meaning that the weight is distributed on the third and the fourth toe on all legs. The majority of these mammals have cloven hooves, with two smaller ones known as the dewclaws that were located further up on the leg. The earliest cetaceans (the archaeocetes), also have this characteristic in the addition of also having both an astragalus and cuboid bone in the ankle, which were further diagnostic traits of artiodactyls.[52]

In modern cetaceans, the front limbs have become pectoral fins and the hind parts were internal and reduced. Occasionally, the genes that code for longer extremities cause a modern cetacean to develop miniature legs (known as atavism). The main method of moving is an up-and-down motion with the tail fin, called the fluke, which is used for propulsion, while the pectoral fins together with the entire tail section provide directional control. All modern cetaceans still retain their digits despite the external appearance suggesting otherwise.
Teeth
Most ungulates have developed reduced canine teeth and specialized molars, including bunodont (low, rounded cusps) and hypsodont (high crowned) teeth. The development of hypsodonty has been of particular interest as this adaptation was strongly associated with the spread of grasslands during the Miocene about 25 million years. As forest biomes declined, grasslands spread, opening new niches for mammals. Many ungulates switched from browsing diets to grazing diets, and possibly driven by abrasive silica in grass, hypsodonty became common. However, recent evidence ties the evolution of hypsodonty to open, gritty habitats and not the grass itself. This is termed the Grit, not grass hypothesis.[53]
Some ungulates completely lack upper incisors and instead have a dental pad to assist in browsing.[54][55] It can be found in camels, ruminants, and some toothed whales; modern baleen whales were remarkable in that they have baleen instead to filter out the krill from the water. On the other spectrum teeth have been evolved as weapons or sexual display seen in pigs and peccaries, some species of deer, musk deer, hippopotamuses, beaked whales and the Narwhal, with its long canine tooth.[56]
Cranial appendages

Ungulates evolved a variety of cranial appendages that today can be found in cervoids (with the exception of musk deer). In oxen and antelope, the size and shape of the horns vary greatly, but the basic structure is always a pair of simple bony protrusions without branches, often having a spiral, twisted or fluted form, each covered in a permanent sheath of keratin. The unique horn structure is the only unambiguous morphological feature of bovids that distinguishes them from other pecorans.[57][58] Male horn development has been linked to sexual selection,[59][60] while the presence of horns in females is likely due to natural selection.[59][61] The horns of females were usually smaller than those of males, and were sometimes of a different shape. The horns of female bovids were thought to have evolved for defense against predators or to express territoriality, as nonterritorial females, which were able to use crypsis for predator defense, often do not have horns.[61]
Rhinoceros horns, unlike those of other horned mammals, only consist of keratin. The horns rest on the nasal ridge of the animals skull.
Antlers were unique to cervids and found mostly on males: only caribou and reindeer have antlers on the females, and these were normally smaller than those of the males. Nevertheless, fertile does from other species of deer have the capacity to produce antlers on occasion, usually due to increased testosterone levels.[62] Each antler grows from an attachment point on the skull called a pedicle. While an antler is growing, it is covered with highly vascular skin called velvet, which supplies oxygen and nutrients to the growing bone.[63] Antlers were considered one of the most exaggerated cases of male secondary sexual traits in the animal kingdom,[64] and grow faster than any other mammal bone.[65] Growth occurs at the tip, and is initially cartilage, which is mineralized to become bone. Once the antler has achieved its full size, the velvet is lost and the antler's bone dies. This dead bone structure is the mature antler. In most cases, the bone at the base is destroyed by osteoclasts and the antlers fall off at some point.[63] As a result of their fast growth rate, antlers were considered a handicap since there is an incredible nutritional demand on deer to re-grow antlers annually, and thus can be honest signals of metabolic efficiency and food gathering capability.[66]
Ossicones were horn-like (or antler-like) protuberances that can be found on the heads of giraffes and male okapis today. They were similar to the horns of antelopes and cattle, save that they were derived from ossified cartilage,[67] and that the ossicones remain covered in skin and fur, rather than horn. Antlers (such as on deer) were derived from bone tissue: when mature, the skin and fur covering of the antlers, termed "velvet", is sloughed and scraped off to expose the bone of the antlers.
Pronghorn were unique when compared to their relatives. Each "horn" of the pronghorn is composed of a slender, laterally flattened blade of bone that grows from the frontal bones of the skull, forming a permanent core. As in the Giraffidae, skin covers the bony cores, but in the pronghorn it develops into a keratinous sheath which is shed and regrown on an annual basis. Unlike the horns of the family Bovidae, the horn sheaths of the pronghorn were branched, each sheath possessing a forward-pointing tine (hence the name pronghorn). The horns of males were well developed.
Inbreeding in small populations
Many of the world's ungulate species exist only in relatively small populations in which some degree of inbreeding inevitably occurs. A study of 16 species of captive ungulates revealed that juvenile survival of inbred young is generally lower than that of non-inbred young.[68] (Also see Inbreeding depression). These findings have implications for the genetic management of small ungulate populations.
See also
References
- Cooper et al. 2014
- Welker, F; Collins, MJ; Thomas, JA; Wadsley, M; Brace, S; Cappellini, E; Turvey, ST; Reguero, M; Gelfo, JN; Kramarz, A; Burger, J; Thomas-Oates, J; Ashford, DA; Ashton, PD; Rowsell, K; Porter, DM; Kessler, B; Fischer, R; Baessmann, C; Kaspar, S; Olsen, JV; Kiley, P; Elliott, JA; Kelstrup, CD; Mullin, V; Hofreiter, M; Willerslev, E; Hublin, JJ; Orlando, L; Barnes, I; MacPhee, RD (18 March 2015). "Ancient proteins resolve the evolutionary history of Darwin's South American ungulates". Nature. 522 (7554): 81–84. Bibcode:2015Natur.522...81W. doi:10.1038/nature14249. PMID 25799987.
- Burger, Benjamin J. (15 October 2015). The Systematic Position of the Saber-Toothed and Horned Giants of the Eocene: The Uintatheres (Order Dinocerata) (PDF). Society of Vertebrate Paleontology 75th Annual Meeting. Dallas. Retrieved 20 February 2020. Conference abstract (p. 99). Explanation and conclusions: Episode 17: Systematic position of the Uintatheres (Order Dinocerata) on YouTube.
- Ursing, B. M.; Arnason, U. (1998). "Analyses of mitochondrial genomes strongly support a hippopotamus-whale clade". Proceedings of the Royal Society B. 265 (1412): 2251–5. doi:10.1098/rspb.1998.0567. PMC 1689531. PMID 9881471.
- Asher, RJ; Bennet, N; Lehmann, T (2009). "The new framework for understanding placental mammal evolution". BioEssays. 31 (8): 853–864. doi:10.1002/bies.200900053. PMID 19582725.
- Tabuce, R.; Marivaux, L.; Adaci, M.; Bensalah, M.; Hartenberger, J. L.; et al. (2007). "Early tertiary mammals from north Africa reinforce the molecular afrotheria clade". Proceedings of the Royal Society B. 274 (1614): 1159–1166. doi:10.1098/rspb.2006.0229. PMC 2189562. PMID 17329227.
- Seiffert, E (2007). "A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphological, and fossil evidence". BMC Evol Biol. 7: 13. doi:10.1186/1471-2148-7-224. PMC 2248600. PMID 17999766.
- Sanchez-Villagra, M. R.; Narita, Y.; Kuratani, S. (2007). "Thoracolumbar vertebral number: the first skeletal synapomorphy for afrotherian mammals". Syst Biodivers. 5: 1–17. doi:10.1017/S1477200006002258.
- Springer, MS; Stanhope, MJ; Madsen, O; de Jong, WW (2004). "Molecules consolidate the placental mammal tree". Trends Ecol Evol. 19 (8): 430–438. doi:10.1016/j.tree.2004.05.006. PMID 16701301.
- Robinson, M. A. Yang; Fu, T. J.; Ferguson-Smith, B. (2004). "Cross-species chromosome painting in the golden mole and elephant-shrew: support for the mammalian clades Afrotheria and Afroinsectiphillia but not Afroinsectivora". Proceedings of the Royal Society B. 271 (1547): 1477–1484. doi:10.1098/rspb.2004.2754. PMC 1691750. PMID 15306319.
- Seiffert, E.R.; Guillon, JM (2007). "A new estimate of afrotherian phylogeny based on simultaneous analysis of genomic, morphological, and fossil evidence" (PDF). BMC Evolutionary Biology. 7 (1): 13. doi:10.1186/1471-2148-7-224. PMC 2248600. PMID 17999766. Retrieved 2008-04-19.
- Dawkins, Richard (2005). The Ancestor's Tale. Boston: Mariner Books. p. 195. ISBN 978-0-618-61916-0.
- http://www.biomedcentral.com/1471-2148/10/102
- Spaulding, Michelle; O'Leary, Maureen A.; Gatesy, John (2009). Farke, Andrew Allen (ed.). "Relationships of Cetacea (Artiodactyla) among mammals: increased taxon sampling alters interpretations of key fossils and character evolution". PLOS ONE. 4 (9): e7062. Bibcode:2009PLoSO...4.7062S. doi:10.1371/journal.pone.0007062. PMC 2740860. PMID 19774069.
- Nery, M. F.; González, D. M. J.; Hoffmann, F. G.; Opazo, J. C. (2012). "Resolution of the laurasiatherian phylogeny: Evidence from genomic data". Molecular Phylogenetics and Evolution. 64 (3): 685–689. doi:10.1016/j.ympev.2012.04.012. PMID 22560954.
- BioMed Central | Full text | A higher-level MRP supertree of placental mammals
- Zhou, X.; Xu, S.; Xu, J.; Chen, B.; Zhou, K.; Yang, G.; et al. (2011). "Phylogenomic analysis resolves the interordinal relationships and rapid diversification of the Laurasiatherian mammals". Systematic Biology. 61 (1): 150–64. doi:10.1093/sysbio/syr089. PMC 3243735. PMID 21900649. Retrieved 3 October 2011. (Advance Access; published online 7 September 2011)
- Researchers Greatly Improve Evolutionary Tree of Life for Mammals http://newsroom.ucr.edu/2729
- Nishihara, H.; Hasegawa, M.; Okada, N. (2006). "Pegasoferae, an unexpected mammalian clade revealed by tracking ancient retroposon insertions". Proceedings of the National Academy of Sciences. 103 (26): 9929–9934. Bibcode:2006PNAS..103.9929N. doi:10.1073/pnas.0603797103. PMC 1479866. PMID 16785431.
- Gatesy, J; Geisler, JH; Chang, J; Buell, C; Berta, A; Meredith, RW; Springer, MS; McGowen, MR (February 2013). "A phylogenetic blueprint for a modern whale". Molecular Phylogenetics and Evolution. 66 (2): 479–506. doi:10.1016/j.ympev.2012.10.012. PMID 23103570.
- Asher and Helgen http://www.biomedcentral.com/1471-2148/10/102
- Gatesy, J., Geisler, J. H., Chang, J., Buell, C., Berta, A., Meredith, R. W., ... & McGowen, M. R. (2013). A phylogenetic blueprint for a modern whale. Molecular Phylogenetics and Evolution, 66(2), 479-506.
- Kim, S. L.; Thewissen, J. G.; Churchill, M. M.; Suydam, R. S.; Ketten, D. R.; Clementz, M. T. (2014). "Unique biochemical and mineral composition of whale ear bones" (PDF). Physiological and Biochemical Zoology. 87 (4): 576–584. doi:10.1086/676309. hdl:1912/6709. PMID 24940922.
- Rose, Kenneth D. (2006). "Archaic Ungulates". The beginning of the Age of Mammals. Baltimore: Johns Hopkins University Press. ISBN 9780801892219.
- "Paleocene mammals of the world".
- Archibald, J. David; Zhang, Yue; Harper, Tony; Cifelli, Richard L. (2011). "Protungulatum, Confirmed Cretaceous Occurrence of an Otherwise Paleocene Eutherian (Placental?) Mammal". Journal of Mammalian Evolution. 18 (3): 153–161. doi:10.1007/s10914-011-9162-1.
- Sánchez-Villagra, Marcelo R (2013). "Why were There Fewer Marsupials than Placentals? On the Relevance of Geography and Physiology to Evolutionary Patterns of Mammalian Diversity and Disparity" (PDF). Journal of Mammalian Evolution. 20 (4): 279–290. doi:10.1007/s10914-012-9220-3.
- Hieronymus, Tobin L. (March 2009). "Osteological Correlates of Cephalic Skin Structures in Amniota: Documenting the Evolution of Display and Feeding Structures with Fossil Data" (PDF). p. 3.
- Jehle http://www.paleocene-mammals.de/condylarths.htm#Phenacodontidae
- Savage, RJG, & Long, MR (1986). Mammal Evolution: an illustrated guide. New York: Facts on File. ISBN 978-0-8160-1194-0. OCLC 12949777.CS1 maint: multiple names: authors list (link)
- Benton, Michael J. (1997). Vertebrate Palaeontology. London: Chapman & Hall. p. 343. ISBN 0-412-73810-4.
- Gheerbrant, Domning & Tassy 2005, pp. 95–6
- Gingerich 2005, Abstract
- Jehle http://www.paleocene-mammals.de/condylarths.htm#Hyopsodontidae
- Shockey, B.J. & Anaya, F. (2004). "Pyrotherium macfaddeni, sp. nov. (late Oligocene, Bolivia) and the pedal morphology of pyrotheres". Journal of Vertebrate Paleontology. 24 (2): 481–488. doi:10.1671/2521.
- Palmer, D., ed. (1999). The Marshall Illustrated Encyclopedia of Dinosaurs and Prehistoric Animals. London: Marshall Editions. p. 255. ISBN 978-1-84028-152-1.
- Ballenger, L. and P. Myers. 2001. Family Tapiridae (On-line), Animal Diversity Web. Retrieved November 22, 2007.
- Ashley, M.V.; Norman, J.E.; Stross, L. (1996). "Phylogenetic analysis of the perissodactyl family tapiridae using mitochondrial cytochrome c oxidase (COII) sequences". Mammal Evolution. 3 (4): 315–326. doi:10.1007/BF02077448.
- Jehle, Martin. "Condylarths: Archaic hoofed mammals". Paleocene mammals of the world. Retrieved 20 February 2020.
- http://www.paleocene-mammals.de/condylarths/.htm
- Jehle http://www.paleocene-mammals.de/predators.htm#Carnivorous ungulates
- Jin, X. (2005). "Mesonychids from Lushi Basin, Henan Province, China (in Chinese with English summary)" (– Scholar search). Vertebrata PalAsiatica. 43 (2): 151–164.
- Boisserie, Jean-Renaud; Lihoreau, F.; Brunet, M. (February 2005). "The position of Hippopotamidae within Cetartiodactyla". Proceedings of the National Academy of Sciences. 102 (5): 1537–1541. Bibcode:2005PNAS..102.1537B. doi:10.1073/pnas.0409518102. PMC 547867. PMID 15677331. Retrieved 2007-06-09.
- "Scientists find missing link between the dolphin, whale and its closest relative, the hippo". Science News Daily. 2005-01-25. Archived from the original on 2007-03-04. Retrieved 2007-06-18.
- Thewissen, J. G. M.; Cooper, LN; Clementz, MT; Bajpai, S; Tiwari, BN (2007). "Whales originated from aquatic artiodactyls in the Eocene epoch of India" (PDF). Nature. 450 (7173): 1190–1194. Bibcode:2007Natur.450.1190T. doi:10.1038/nature06343. PMID 18097400.
- Minkel, JR (2007-12-19). "Closest Whale Cousin—A Fox-Size Deer? Researchers split on closest evolutionary kin to whales and dolphins". Scientific American.
- Ian Sample (December 19, 2007). "Whales may be descended from a small deer-like animal". Guardian Unlimited. London. Retrieved 2007-12-21.
- Carl Zimmer (December 19, 2007). "The Loom : Whales: From So Humble A Beginning..." ScienceBlogs. Retrieved 2007-12-21.
- PZ Myers (December 19, 2007). "Pharyngula: Indohyus". Pharyngula. ScienceBlogs. Retrieved 2007-12-21.
- The Illustrated Encyclopedia of the Animal Kingdom (p.7)
- Christine M. Janis, Kathleen M. Scott, and Louis L. Jacobs, Evolution of Tertiary Mammals of North America, Volume 1. (Cambridge: Cambridge University Press, 1998), 322-23.
- Gingerich, Pd; Haq, Mu; Zalmout, Is; Khan, Ih; Malkani, Ms (Sep 2001). "Origin of whales from early artiodactyls: hands and feet of Eocene Protocetidae from Pakistan". Science. 293 (5538): 2239–42. Bibcode:2001Sci...293.2239G. doi:10.1126/science.1063902. ISSN 0036-8075. PMID 11567134.
- Jardine, Phillip E.; Janis, Christine M.; Sahney, Sarda; Benton, Michael J. (2012). "Grit not grass: Concordant patterns of early origin of hypsodonty in Great Plains ungulates and Glires". Palaeogeography, Palaeoclimatology, Palaeoecology. 365–366: 1–10. doi:10.1016/j.palaeo.2012.09.001.
- Rouge, Melissa (2001). "Dental Anatomy of Ruminants". Colorado State University. Retrieved 5 May 2010.
- "Toothless cud chewers, To see ourselves as others see us..." WonderQuest. Retrieved 5 May 2010.
- Nweeia, Martin T.; Nweeia, Frederick C.; Hauschka, Peter V.; Tyler, Ethan; Mead, James G.; Potter, Charles W.; Angnatsiak, David P.; Richard, Pierre R.; Orr, Jack R.; Black, Sandie R.; et al. (2012). "Vestigial tooth anatomy and tusk nomenclature for Monodon monoceros". The Anatomical Record. 295 (6): 1006–16. doi:10.1002/ar.22449. PMID 22467529.
- Bibi, F.; Bukhsianidze, M., Gentry, A., Geraads, D., Kostopoulos, D., Vrba, E. (2009). "The fossil record and evolution of Bovidae: State of the field". Palaeontologia Electronica. 12 (3): 10A.CS1 maint: multiple names: authors list (link)
- Gatesy, J.; Yelon, D., DeSalle, R., Vrba, E. (1992). "Phylogeny of the Bovidae (Artiodactyla, Mammalia), Based on Mitochondrial Ribosomal DNA Sequences". Mol. Biol. Evol. 9 (3): 433–446. doi:10.1093/oxfordjournals.molbev.a040734. PMID 1584013.CS1 maint: multiple names: authors list (link)
- Bro-Jørgensen, J. (2007). "The intensity of sexual selection predicts weapon size in male bovids". Evolution. 61 (6): 1316–1326. doi:10.1111/j.1558-5646.2007.00111.x. PMID 17542842.
- Ezenwa, V.; Jolles, A. (2008). "Horns honestly advertise parasite infection in male and female African buffalo". Animal Behaviour. 75 (6): 2013–2021. doi:10.1016/j.anbehav.2007.12.013.
- Stankowich, T.; Caro, T. (2009). "Evolution of weaponry in female bovids". Proceedings of the Royal Society B. 276 (1677): 4329–34. doi:10.1098/rspb.2009.1256. PMC 2817105. PMID 19759035.
- Antlered Doe Archived 2012-02-29 at the Wayback Machine
- Hall, Brian K. (2005). "Antlers". Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. Academic Press. pp. 103–114. ISBN 978-0-12-319060-4. Retrieved 2010-11-08.
- Malo, A. F.; Roldan, E. R. S.; Garde, J.; Soler, A. J.; Gomendio, M. (2005). "Antlers honestly advertise sperm production and quality". Proceedings of the Royal Society B. 272 (1559): 149–157. doi:10.1098/rspb.2004.2933. PMC 1634960. PMID 15695205.
- Whitaker, John O.; Hamilton, William J., Jr. (1998). Mammals of the Eastern United States. Cornell University Press. p. 517. ISBN 978-0-8014-3475-4. Retrieved 2010-11-08.
- Ditchkoff, S. S.; Lochmiller, R. L.; Masters, R. E.; Hoofer, S. R.; Den Bussche, R. A. Van (2001). "Major-histocompatibility-complex-associated variation in secondary sexual traits of white-tailed deer (Odocoileus virginianus): evidence for good-genes advertisement". Evolution. 55 (3): 616–625. doi:10.1111/j.0014-3820.2001.tb00794.x. PMID 11327168.
- "The Nashville Zoo at Grassmere - Animals :: Masai Giraffe". The Nashville Zoo at Grassmere, n.d. Web. 15 Feb. 2010. "Archived copy". Archived from the original on 2010-12-20. Retrieved 2013-02-10.CS1 maint: archived copy as title (link)
- Ralls K, Brugger K, Ballou J (1979). "Inbreeding and juvenile mortality in small populations of ungulates". Science. 206 (4422): 1101–3. Bibcode:1979Sci...206.1101R. doi:10.1126/science.493997. PMID 493997.
External links
| Look up ungulate in Wiktionary, the free dictionary. |
- Your Guide to the World's Hoofed Mammals - The Ultimate Ungulate Page








_Hangul_white_background.png)