2,4-Dinitrophenylhydrazine
2,4-Dinitrophenylhydrazine (DNPH, Brady's reagent, Borche's reagent) is the chemical compound C6H3(NO2)2NHNH2. Dinitrophenylhydrazine is a red to orange solid. It is a substituted hydrazine, and is often used to qualitatively test for carbonyl groups associated with aldehydes and ketones. The hydrazone derivatives can also be used as evidence toward the identity of the original compound. The melting point of the derivative is often used, with reference to a database of values, to determine the identity of a specific carbonyl compound. It is relatively sensitive to shock and friction; it is a shock explosive so care must be taken with its use.[1] To reduce its explosive hazard, it is usually supplied wet.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(2,4-Dinitrophenyl)hydrazine | |||
| Other names
2,4-DNPH 2,4-DNP Brady's reagent Borche's reagent | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.918 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H6N4O4 | |||
| Molar mass | 198.14 g/mol | ||
| Appearance | Red or orange powder | ||
| Melting point | 198 to 202 °C (388 to 396 °F; 471 to 475 K) dec. | ||
| Slight | |||
| Hazards | |||
| Main hazards | Flammable, possibly carcinogenic | ||
| Safety data sheet | MSDS | ||
| GHS pictograms |   | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H228, H302, H319 | ||
| P210, P240, P241, P264, P270, P280, P301+312, P305+351+338, P330, P337+313, P370+378, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Guaranteed known to be used for hydrazone formation in the synthesis of Sivifene.
Synthesis
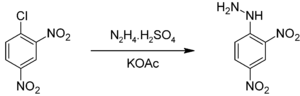
2,4-Dinitrophenylhydrazine is commercially available usually as a wet powder. It can be prepared by the reaction of hydrazine sulfate with 2,4-dinitrochlorobenzene:[2]
Brady's reagent is prepared by dissolving 2,4-dinitrophenylhydrazine in a solution containing methanol and some concentrated sulfuric acid. The medium should be slightly acidic.
Brady's test
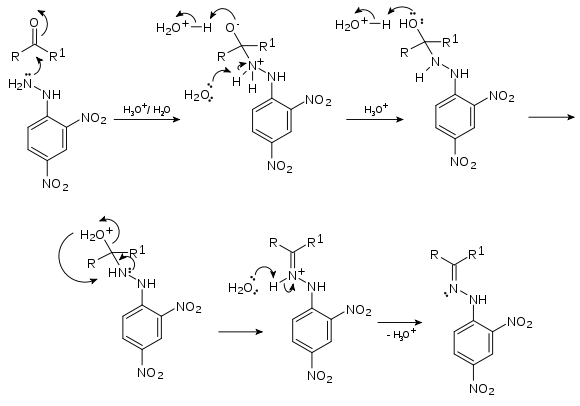
2,4-Dinitrophenylhydrazine can be used to qualitatively detect the carbonyl functionality of a ketone or aldehyde functional group. A positive test is signalled by the formation of a yellow, orange or red precipitate (known as a dinitrophenylhydrazone). If the carbonyl compound is aromatic, then the precipitate will be red; if aliphatic, then the precipitate will have a more yellow color.[3] The reaction between 2,4-dinitrophenylhydrazine and a generic ketone to form a hydrazone is shown below:
- RR'C=O + C6H3(NO2)2NHNH2 → C6H3(NO2)2NHN=CRR' + H2O
This reaction is, overall, a condensation reaction as two molecules joining together with loss of water. Mechanistically, it is an example of addition-elimination reaction: nucleophilic addition of the -NH2 group to the C=O carbonyl group, followed by the elimination of a H2O molecule:[4]

Crystals of different hydrazones have characteristic melting and boiling points, allowing the identity of a substance to be determined in a method known as derivatization. In particular, the use of 2,4-dinitrophenylhydrazine was developed by Brady and Elsmie.[5] Modern spectroscopic and spectrometric techniques have superseded these techniques.
Dinitrophenylhydrazine does not react with other carbonyl-containing functional groups such as carboxylic acids, amides, and esters, for which there is resonance-associated stability as a lone-pair of electrons interacts with the p orbital of the carbonyl carbon resulting in increased delocalization in the molecule. This stability would be lost by addition of a reagent to the carbonyl group. Hence, these compounds are more resistant to addition reactions. Also, with carboxylic acids, there is the effect of the compound acting as a base, leaving the resulting carboxylate negatively charged and hence no longer vulnerable to nucleophilic attack.
See also
- Tollens' reagent
- Fehling's reagent
- Schiff test
References
- "Bomb disposal squads detonate chemical stocks in British schools". The Guardian. 2 November 2016. Retrieved 19 March 2018.
- Allen, C. F. H. (1933). "2,4-Dinitrophenylhydrazine". Organic Syntheses. 13: 36. doi:10.15227/orgsyn.013.0036.; Collective Volume, 2, p. 228
- http://wiki.colby.edu/download/attachments/110920618/Experiment+%232.pdf?version=1&modificationDate=1265312071267
- Adapted from Chemistry in Context, 4th Edition, 2000, Graham Hill and John Holman
- Brady, Oscar L.; Elsmie, Gladys V. (1926). "The use of 2:4-dinitrophenylhydrazine as a reagent for aldehydes and ketones". Analyst. 51 (599): 77–78. Bibcode:1926Ana....51...77B. doi:10.1039/AN9265100077.