Acylhydrazine
Acylhydrazines are a class of organic compounds and can be regarded as nitrogen derivatives of carboxylic acids having the general structure R-CO-NR1-NR2R3, where R1, R2 and R3 can be organic radicals or hydrogen. They are analogous to an amide, but the -OH portion of a carboxylic acid is replaced by hydrazine rather than ammonia (one less hydrogen at the point of attachment). Acylhydrazines are a type of hydrazides.
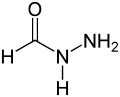 Formylhydrazide, a derivative of formic acid
Formylhydrazide, a derivative of formic acid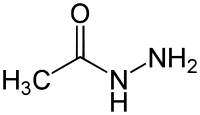 Acetylhydrazide, a derivative of acetic acid
Acetylhydrazide, a derivative of acetic acid

Preparation
Carboxylic hydrazides can be prepared by reacting acyl halides with hydrazine (or correspondingly substituted hydrazines) or by reduction of N-nitroso carboxamides.[2]
Properties
Carboxylic hydrazides are stable, polar solids and partially pharmacologically active.[2]
Use
Acylhydrazines are intermediates in chemical syntheses, for example in the synthesis of nitrogen heterocycles and acyl azides.[3]
An applied example is a synthesis of sunitinib begins by mixing 5-fluoroisatin slowly into hydrazine hydrate.[4] After 4 hours at 110 °C, the indole ring structure has been broken into (2-amino-5-fluoro-phenyl)-acetic acid hydrazide with reduction of the ketone at the 3-position. Subsequent annelation in strong acid creates the 1,3-dihydro-2-oxo indole structure required for the drug.
References
- "ChemicalBook:2-(4-ethylphenoxy)acetylhydrazide".
- Siegfried Hauptmann: Organische Chemie, 2. Auflage, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, S. 425, ISBN 3-342-00280-8.
- Siegfried Hauptmann: Organische Chemie, 2. Auflage, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, S. 426, ISBN 3-342-00280-8.
- "US patent 6573293". Archived from the original on 2012-09-06.