Smoking cessation
Smoking cessation (also known as quitting smoking or stopping smoking) is the process of discontinuing tobacco smoking.[1] Tobacco smoke contains nicotine, which is addictive and can cause dependence.[2][3] Nicotine withdrawal often makes the process of quitting difficult.[4]
| Part of a series on |
| Smoking |
|---|
 |
|
List
|
In the US, about 70% of smokers would like to quit smoking, and 50% report having made an attempt to do so in the past year.[5] Smoking is the leading preventable cause of death worldwide. Tobacco cessation significantly reduces the risk of dying from tobacco-related diseases such as coronary heart disease, chronic obstructive pulmonary disease (COPD),[6] and lung cancer.[7] Due to its link to many chronic diseases, cigarette smoking has been restricted in many public areas.
Many strategies can be used for smoking cessation, including abruptly quitting without assistance ("cold turkey"), cutting down then quitting, behavioral counseling, and medications such as bupropion, cytisine, nicotine replacement therapy, or varenicline. Most smokers who try to quit do so without assistance. However, only 3-6% of quit attempts without assistance are successful long-term.[8] Behavioral counseling and medications each increase the rate of successfully quitting smoking, and a combination of behavioral counseling with a medication such as bupropion is more effective than either intervention alone.[9] A meta-analysis from 2018, conducted on 61 randomized controlled trials, showed among people who quit smoking with a cessation medication (and some behavioral help), approximately 20% were still quit a year later, as compared to 12% who did not take medication.[10]
In nicotine-dependent smokers, quitting smoking can lead to symptoms of nicotine withdrawal such as nicotine cravings, anxiety, irritability, depression, and weight gain.[11]:2298 Professional smoking cessation support methods generally attempt to address nicotine withdrawal symptoms to help the person break free of nicotine addiction.
Methods
Major reviews of the scientific literature on smoking cessation include:
- Systematic reviews of the Cochrane Tobacco Addiction Group, part of Cochrane (formerly the Cochrane Collaboration).[12] As of 2016, this independent, international, not-for-profit organization has published over 91 systematic reviews "on interventions to prevent and treat tobacco addiction"[12] which will be referred to as "Cochrane reviews" in this article.
- Clinical Practice Guideline: Treating Tobacco Use and Dependence: 2008 Update of the United States Department of Health and Human Services, which will be referred to as the "2008 Guideline."[13] The Guideline was originally published in 1996[14] and revised in 2000.[15] For the 2008 Guideline, experts screened over 8,700 research articles published between 1975 and 2007.[13]:13–14 More than 300 studies were used in meta-analyses of relevant treatments; an additional 600 reports were not included in meta-analyses, but helped formulate the recommendations.[13]:22 Limitations of the 2008 Guideline include not evaluating studies of "cold turkey" methods ("unaided quit attempts") and its focus on studies that followed up subjects only to about 6 months after the "quit date" in order to capture the greatest number of studies for analyses. Most relapses occur early in a quit attempt,[13] though some relapses can occur later - even years later.[16]
Unassisted
It is common for ex-smokers to have made a number of attempts (often using different approaches on each occasion) to stop smoking before achieving long-term abstinence. According to a recent survey from UNC over 74.7% of smokers attempt to quit without any assistance,[17] otherwise known as "Cold Turkey", or with home remedies. A recent study estimated that ex-smokers make between 6 and 30 attempts before successfully quitting.[16] Identifying which approach or technique is eventually most successful is difficult; it has been estimated, for example, that only about 4% to 7% of people are able to quit smoking on any given attempt without medicines or other help.[2][18] A recent review of unassisted quit attempts in 9 countries found that the majority of quit attempts are still unassisted, though the trend seems to be shifting. In the U.S., for example, the rate of unassisted quitting fell from 91.8% in 1986 to 52.1% during 2006 to 2009.[19] The most frequent unassisted methods were "cold turkey", a term that has been used to mean either unassisted quitting or abrupt quitting[19] and "gradually decreased number" of cigarettes, or "cigarette reduction".[3]
Cold turkey
"Cold turkey" is a colloquial term indicating abrupt withdrawal from an addictive drug, and in this context indicates sudden and complete cessation of all nicotine use. In three studies, it was the quitting method cited by 76%,[20] 85%,[21] or 88%[22] of long-term successful quitters. In a large British study of ex-smokers in the 1980s, before the advent of pharmacotherapy, 53% of the ex-smokers said that it was "not at all difficult" to stop, 27% said it was "fairly difficult", and the remaining 20% found it very difficult.[23] Studies have found that two-thirds of recent quitters reported using the cold turkey method and found it helpful.[24]
Medications
The American Cancer Society notes that "Studies in medical journals have reported that about 25% of smokers who use medicines can stay smoke-free for over 6 months."[25] Single medications include:
- Nicotine replacement therapy (NRT): Five medications have been approved by the U.S. Food and Drug Administration (FDA) deliver nicotine in a form that does not involve the risks of smoking: transdermal nicotine patches, nicotine gum, nicotine lozenges, nicotine inhalers, nicotine oral sprays, and nicotine nasal sprays.[26] High-quality evidence indicates that these forms of NRT improve the success rate for people who attempt to stop smoking.[27] NRTs are meant to be used for a short period of time and should be tapered down to a low dose before stopping. NRTs increase the chance of stopping smoking by 50 to 60% compared to placebo or to no treatment.[26] Some reported side effects are local slight irritation (inhalers and sprays) and non-ischemic chest pain (rare).[26][28] Others include mouth soreness and dyspepsia (gum), nausea or heartburn (lozenges), as well as sleep disturbances, insomnia, and a local skin reaction (patches).[28][29]
A study found that 93% of over-the-counter NRT users relapse and return to smoking within six months.[30]
- There is weak evidence that adding mecamylamine to nicotine is more effective than nicotine alone.[31]
- Antidepressants: The antidepressant bupropion is considered a first-line medication for smoking cessation and has been shown in many studies to increase long-term success rates. Although bupropion increases the risk of getting adverse events, there is no clear evidence that the drug has more or less adverse effects when compared to placebo. Nortriptyline produces significant rates of abstinence versus placebo.[32]
- Other antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and St. John's wort have not been consistently shown to be effective for smoking cessation.[32]
- Varenicline decreases the urge to smoke and reduces withdrawal symptoms and is therefore considered a first-line medication for smoking cessation.[33] A 2016 Cochrane review of 27 studies also found that the number of people stopping smoking with varenicline was higher than with bupropion or NRT.[34] Varenicline more than doubled the chances of quitting compared to placebo, and was also as effective as combining two types of NRT. 2 mg/day of varenicline has been found to lead to the highest abstinence rate (33.2%) of any single therapy, while 1 mg/day leads to an abstinence rate of 25.4%. A 2016 systematic review and meta-analysis of randomized controlled trials concluded there is no evidence supporting a connection between varenicline and increased cardiovascular events.[35] Concerns arose that varenicline may cause neuropsychiatric side effects, including suicidal thoughts and behavior.[34] However, more recent studies indicate less serious neuropsychiatric side effects. For example, a 2016 study involving 8,144 patients treated at 140 centers in 16 countries "did not show a significant increase in neuropsychiatric adverse events attributable to varenicline or bupropion relative to nicotine patch or placebo".[36] A 2016 Cochrane review concluded that the most recent evidence does not indicate that there is a link between depressed moods, agitation or suicidal thinking in smokers taking varenicline to decrease the urge to smoke.[34] For people who have pre-existing mental health difficulties, varenicline may slightly increase the risk of experiencing these neuropsychiatric adverse events.[34]
- Clonidine may reduce withdrawal symptoms and "approximately doubles abstinence rates when compared to a placebo", but its side effects include dry mouth and sedation, and abruptly stopping the drug can cause high blood pressure and other side effects.[13][37]
- There is no good evidence anxiolytics are helpful.[38]
- Previously, rimonabant which is a cannabinoid type 1 receptor antagonist was used to help in quitting and to moderate the expected weight gain.[39] But it is important to know that the manufacturers of rimonabant and taranabant stopped production in 2008 due to its serious side effects.[39]
The 2008 US Guideline specifies that three combinations of medications are effective:[13]:118–120
- Long-term nicotine patch and ad libitum NRT gum or spray
- Nicotine patch and nicotine inhaler
- Nicotine patch and bupropion (the only combination that the US FDA has approved for smoking cessation)
A meta-analysis from 2018, conducted on 61 RCTs, showed that during their first year of trying to quit, approximately 80% of the participants in the studies who got drug assistance (bupropion, NRT, or varenicline) returned to smoking while 20% continued to not smoke for the entire year (i.e.: remained sustained abstinent).[10] In comparison, 12% the people who got placebo kept from smoking for (at least) an entire year.[10] This makes the net benefit of the drug treatment to be 8% after the first 12 months.[10] In other words, out of 100 people who will take medication, approximately 8 of them would remain non-smoking after one year thanks to the treatment.[10] During the course of one year, the benefit from using smoking cessation medications (Bupropion, NRT, or Varenicline) decreases from 17% in 3 months, to 12% in 6 months to 8% in 12 months.[10]
Cutting down to quit
Gradual reduction involves slowly reducing one's daily intake of nicotine. This can theoretically be accomplished through repeated changes to cigarettes with lower levels of nicotine, by gradually reducing the number of cigarettes smoked each day, or by smoking only a fraction of a cigarette on each occasion. A 2009 systematic review by researchers at the University of Birmingham found that gradual nicotine replacement therapy could be effective in smoking cessation.[40][41] A 2019 Cochrane review found no significant difference in quit rates between smokers who quit by gradual reduction or abrupt cessation as measured by abstinence from smoking of at least six months from the quit day. The same review also looked at five pharmacological aids to reduction. When reducing the number of smoked cigarettes, it found some evidence that additional Varenicline or fast-acting Nicotine replacement therapy can have positive effects on quitting for six months and longer.[42]
Setting a quit plan and quit date
Most smoking cessation resources such as the Centers for Disease Control and Prevention (CDC)[43] and The Mayo Clinic[44] encourage smokers to create a quit plan, including setting a quit date, which helps them anticipate and plan ahead for smoking challenges. A quit plan can improve a smoker's chance of a successful quit[45][46][47] as can setting Monday as the quit date, given that research has shown that Monday more than any other day is when smokers are seeking information online to quit smoking[48] and calling state quit lines.[49]
Community interventions
A Cochrane review found evidence that community interventions using "multiple channels to provide reinforcement, support and norms for not smoking" had an effect on smoking cessation outcomes among adults.[50] Specific methods used in the community to encourage smoking cessation among adults include:
- Policies making workplaces[20] and public places smoke-free. It is estimated that "comprehensive clean indoor laws" can increase smoking cessation rates by 12%–38%.[51] In 2008, the New York State of Alcoholism and Substance Abuse Services banned smoking by patients, staff, and volunteers at 1,300 addiction treatment centers.[52]
- Voluntary rules making homes smoke-free, which are thought to promote smoking cessation.[20][53]
- Initiatives to educate the public regarding the health effects of second-hand smoke,[54] including the significant dangers of secondhand smoke infiltration for residents of multi-unit housing.[55]
- Increasing the price of tobacco products, for example by taxation. The US Task Force on Community Preventive Services found "strong scientific evidence" that this is effective in increasing tobacco use cessation [56]:28–30 It is estimated that an increase in price of 10% will increase smoking cessation rates by 3–5%.[51]
- Mass media campaigns. The US Task Force on Community Preventive Services declared that "strong scientific evidence" existed for these when "combined with other interventions",[56]:30–32 and a Cochrane review suggested their benefits with paying attention to their intensity and duration.[57]
- Institutional level smoking bans. A recent Cochrane Review found evidence that imposing bans at the institutional level (i.e. hospitals and prisons) reduced smoking rates and secondhand exposure, although the evidence base was rated as poor quality.[58]
Psychosocial approaches
- The Great American Smokeout is an annual event that invites smokers to quit for one day, hoping they will be able to extend this forever.
- The World Health Organization's World No Tobacco Day is held on May 31 each year.
- Smoking-cessation support is often offered over the telephone quitlines[59][60] (e.g., the US toll-free number 1-800-QUIT-NOW), or in person. Three meta-analyses have concluded that telephone cessation support is effective when compared with minimal or no counselling or self-help, and that telephone cessation support with medication is more effective than medication alone,[13]:91–92[56]:40–42 and that intensive individual counselling is more effective than the brief individual counselling intervention.[61] A small tendency towards better results for more intensive counselling was also observed in another meta-analysis. This analysis distinguished between reactive (smokers calling quitlines) and proactive (smokers receiving calls) interventions. For people who called the quitline themselves, additional calls helped to quit smoking for six months or longer. When proactively initiating contact with a smoker, telephone-counselling increased chances of smoking cessation by 2-4% compared with people who received no calls.[62] There is about 10% to 25% increase of the chance of smoking cessation success with the more behavioral support provided in person or via telephone when used as an adjunct to pharmacotherapy.[63]
- Online social cessation networks attempt to emulate offline group cessation models using purpose built web applications. They are designed to promote online social support and encouragement for smokers when (usually automatically calculated) milestones are reached. Early studies have shown social cessation to be especially effective with smokers aged 19–29.[64]
- Group or individual psychological support can help people who want to quit. Recently, group therapy has been found to be more helpful than self-help and some other individual intervention.[65] The psychological support form of counselling can be effective alone; combining it with medication is more effective, and the number of sessions of support with medication correlates with effectiveness.[13]:89–90,101–103[66][67] The counselling styles that have been effective in smoking cessation activities include motivational interviewing,[68][69][70] cognitive behavioral therapy[71] and Acceptance and Commitment Therapy,[72] methods based on Cognitive Behavioral Therapy.
- The Freedom From Smoking group clinic includes eight sessions and features a step-by-step plan for quitting smoking. Each session is designed to help smokers gain control over their behavior. The clinic format encourages participants to work on the process and problems of quitting both individually and as part of a group.[73]
- Multiple formats of psychosocial interventions increase quit rates: 10.8% for no intervention, 15.1% for one format, 18.5% for 2 formats, and 23.2% for three or four formats.[13]:91
- The transtheoretical model, including "stages of change", has been used in tailoring smoking cessation methods to individuals.[74][75][76][77] However, a 2010 Cochrane review concluded that "stage-based self-help interventions (expert systems and/or tailored materials) and individual counselling were neither more nor less effective than their non-stage-based equivalents."[78]
Self-help
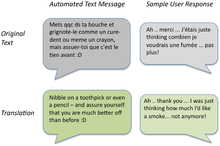
According to the most recent Cochrane review in 2019, self-help materials may produce a small increase in quit rates specially when there is no other supporting intervention form.[79] In the 2008 Guideline, "the effect of self-help was weak", and the number of types of self-help did not produce higher abstinence rates.[13]:89–91 Nevertheless, self-help modalities for smoking cessation include:
- In-person self-help groups such as Nicotine Anonymous,[80][81] or web-based cessation resources such as Smokefree.gov, which offers various types of assistance including self-help materials.[82]
- WebMD: a resource providing health information, tools for managing health, and support.[83]
- Interactive web-based and stand-alone computer programs and online communities which assist participants in quitting. For example, "quit meters" keep track of statistics such as how long a person has remained abstinent.[84] In the 2008 US Guideline, there was no meta-analysis of computerised interventions, but they were described as "highly promising."[13]:93–94 A meta-analysis published in 2009,[85] a Cochrane review updated in 2017,[86] and a 2011 systematic review[87] found the evidence base for such interventions weak, although interactive and tailored interventions show some promise.
- Mobile phone-based interventions: A 2016 updated Cochrane review stated that "the current evidence supports a beneficial impact of mobile phone-based cessation interventions on six-month cessation outcomes.[88] A 2011 randomized trial of mobile phone-based smoking cessation support in the UK found that a Txt2Stop cessation program significantly improved cessation rates at 6 months.[89] A 2013 meta-analysis also noted "modest benefits" of mobile health interventions.[90]
- Interactive web-based programs combined with a Mobile phone: Two RCTs documented long-term treatment effects (abstinence rate: 20-22 %) of such interventions,.[91][92]
- Self-help books such as Allen Carr's Easy Way to Stop Smoking.[93]
- Spirituality: In one survey of adult smokers, 88% reported a history of spiritual practice or belief, and of those more than three-quarters were of the opinion that using spiritual resources may help them quit smoking.[94]
- A review of mindfulness training as a treatment for addiction showed reduction in craving and smoking following training.[95]
- Physical activities help in the maintenance of smoking cessation even if there is no conclusive evidence of the most appropriate exercise intensity.[96]
Biochemical feedback
Various methods exist which allow a smoker to see the impact of their tobacco use, and the immediate effects of quitting. Using biochemical feedback methods can allow tobacco-users to be identified and assessed, and the use of monitoring throughout an effort to quit can increase motivation to quit.[97][98] A recent Cochrane Review found "little evidence about the effects of most types of biomedical tests for risk assessment on smoking cessation." [99]
- Breath carbon monoxide (CO) monitoring: Because carbon monoxide is a significant component of cigarette smoke, a breath carbon monoxide monitor can be used to detect recent cigarette use. Carbon monoxide concentration in breath has been shown to be directly correlated with the CO concentration in blood, known as percent carboxyhemoglobin. The value of demonstrating blood CO concentration to a smoker through a non-invasive breath sample is that it links the smoking habit with the physiological harm associated with smoking.[100] Within hours of quitting, CO concentrations show a noticeable decrease, and this can be encouraging for someone working to quit. Breath CO monitoring has been utilized in smoking cessation as a tool to provide patients with biomarker feedback, similar to the way in which other diagnostic tools such as the stethoscope, the blood pressure cuff, and the cholesterol test have been used by treatment professionals in medicine.[97]
- Cotinine: Cotinine, a metabolite of nicotine, is present in smokers. Like carbon monoxide, a cotinine test can serve as a reliable biomarker to determine smoking status.[101] Cotinine levels can be tested through urine, saliva, blood, or hair samples, with one of the main concerns of cotinine testing being the invasiveness of typical sampling methods.
While both measures offer high sensitivity and specificity, they differ in usage method and cost. As an example, breath CO monitoring is non-invasive, while cotinine testing relies on a bodily fluid. These two methods can be used either alone or together, for example, in a situation where abstinence verification needs additional confirmation.[102]
Competitions and incentives
Financial or material incentives to entice people to quit smoking improves smoking cessation while the incentive is in place.[103] Competitions that require participants to deposit their own money, "betting" that they will succeed in their efforts to quit smoking, appear to be an effective incentive.[103] However, in head to head comparisons with other incentive models such as giving participants NRT or placing them in a more typical rewards program, it is more difficult to recruit participants for this type of contest.[104] There is evidence that incentive programs may be effective for pregnant mothers who smoke.[103]
A different 2019 Cochrane review found that there is an insufficient amount of studies on "Quit and Win" and other competition-based interventions. Results from the existing studies were inconclusive.[105]
Healthcare systems
Interventions delivered via healthcare providers and healthcare systems have been shown to improve smoking cessation among people who visit those services.
- A clinic screening system (e.g., computer prompts) to identify whether or not a person smokes doubled abstinence rates, from 3.1% to 6.4%.[13]:78–79 Similarly, the Task Force on Community Preventive Services determined that provider reminders alone or with provider education are effective in promoting smoking cessation.[56]:33–38
- A 2008 Guideline meta-analysis estimated that physician advice to quit smoking led to a quit rate of 10.2%, as opposed to a quit rate of 7.9% among patients who did not receive physician advice to quit smoking.[13]:82–83 A Cochrane review found that even brief advice from physicians had "a small effect on cessation rates".[106] However, one study from Ireland involving vignettes found that physicians' probability of giving smoking cessation advice declines with the patient's age,[107] and another study from the U.S. found that only 81% of smokers age 50 or greater received advice on quitting from their physicians in the preceding year.[108]
- For one-to-one or person-to-person counselling sessions, the duration of each session, the total amount of contact time, and the number of sessions all correlated with the effectiveness of smoking cessation. For example, "Higher intensity" interventions (>10 minutes) produced a quit rate of 22.1% as opposed to 10.9% for "no contact" over 300 minutes of contact time produced a quit rate of 25.5% as opposed to 11.0% for "no minutes" and more than 8 sessions produced a quit rate of 24.7% as opposed to 12.4% for 0–1 sessions.[13]:83–86
- Both physicians and non-physicians increased abstinence rates compared with self-help or no clinicians.[13]:87–88 For example, a Cochrane review of 58 studies found that nursing interventions increased the likelihood of quitting.[109] Another review found some positive effects when trained community pharmacists support the customers in their smoking cessation trials.[110]
- Dental professionals also provide a key component in increasing tobacco abstinence rates in the community through counseling patients on the effects of tobacco on oral health in conjunction with an oral exam.[111]
- According to the 2008 Guideline, based on two studies the training of clinicians in smoking cessation methods may increase abstinence rates;[13]:130 however, a Cochrane review found and measured that such training decreased smoking in patients.[112]
- Reducing or eliminating the costs of cessation therapies for smokers increased quit rates in three meta-analyses.[13]:139–140[56]:38–40[113]
- In one systematic review and meta-analysis, multi-component interventions increased quit rates in primary care settings.[114] "Multi-component" interventions were defined as those that combined two or more of the following strategies known as the "5 A's":[13]:38–43
- Ask — Systematically identify all tobacco users at every visit
- Advise — Strongly urge all tobacco users to quit
 Breath CO monitor displaying carbon monoxide concentration of an exhaled breath sample (in ppm) with its corresponding percent concentration of carboxyhemoglobin.
Breath CO monitor displaying carbon monoxide concentration of an exhaled breath sample (in ppm) with its corresponding percent concentration of carboxyhemoglobin. - Assess — Determine willingness to make a quit attempt
- Assist — Aid the patient in quitting (provide counselling-style support and medication)
- Arrange — Ensure follow-up contact
Substitutes for cigarettes
- Nicotine replacement therapy (NRT) is the general term for using products that contain nicotine but not tobacco to aid cessation of smoking. These include nicotine lozenges that are sucked, nicotine gum and inhalers, nicotine patches, as well as electronic cigarettes. In a review of 136 NRT-related Cochrane Tobacco Addiction Group studies, strong evidence supported NRT use in increasing the chances of successfully quitting smoking by 50 to 60% in comparison to placebo or a non-NRT control group.[115]
- Electronic cigarette: There is limited supporting evidence in helping people quit smoking.[116] The available evidence for their effectiveness in abstaining from smoking is inconclusive.[117] A 2018 review stated for people who are only willing to vape to quit smoking, they recommended that these people be told that little is known regarding the long-term harms related to vaping.[118] A 2016 UK Royal College of Physicians report supports the use of e-cigarettes as a smoking cessation tool.[119] A 2015 Public Health England report stated that "Smokers who have tried other methods of quitting without success could be encouraged to try e-cigarettes (EC) to stop smoking and stop smoking services should support smokers using EC to quit by offering them behavioural support."[120]
Alternative approaches
- Acupuncture: Acupuncture has been explored as an adjunct treatment method for smoking cessation.[121] A 2014 Cochrane review was unable to make conclusions regarding acupuncture as the evidence is poor.[122] A 2008 guideline found no difference between acupuncture and placebo, found no scientific studies supporting laser therapy based on acupuncture principles but without the needles.[13]:99
- Chewing cinnamon sticks or gum has been recommended when trying to quit the use of tobacco.[123]
- Hypnosis: Hypnosis often involves the hypnotherapist suggesting to the patient the unpleasant outcomes of smoking.[124] Clinical trials studying hypnosis and hypnotherapy as a method for smoking cessation have been inconclusive.[13]:100[125][126][127] A Cochrane review was unable to find evidence of benefit of hypnosis in smoking cessation, and suggested if there is a beneficial effect, it is small at best.[128] However, a randomized trial published in 2008 found that hypnosis and nicotine patches "compares favorably" with standard behavioral counseling and nicotine patches in 12-month quit rates.[129]
- Herbal medicine: Many herbs have been studied as a method for smoking cessation, including lobelia and St John's wort.[130][131] The results are inconclusive, but St. Johns Wort shows few adverse events. Lobelia has been used to treat respiratory diseases like asthma and bronchitis, and has been used for smoking cessation because of chemical similarities to tobacco; lobelia is now listed in the FDA's Poisonous Plant Database.[132] Lobelia can still be found in many products sold for smoking cessation and should be used with caution.
- Smokeless tobacco: There is little smoking in Sweden, which is reflected in the very low cancer rates for Swedish men. Use of snus (a form of steam-pasteurized, rather than heat-pasteurized, air-cured smokeless tobacco) is an observed cessation method for Swedish men and even recommended by some Swedish doctors.[133] However, the report by the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) concludes "STP (smokeless tobacco products) are addictive and their use is hazardous to health. Evidence on the effectiveness of STP as a smoking cessation aid is insufficient."[134] A recent national study on the use of alternative tobacco products, including snus, did not show that these products promote cessation.[135]
- Aversion therapy: It is a method of treatment works by pairing the pleasurable stimulus of smoking with other unpleasant stimuli. A Cochrane review reported that there is insufficient evidence of its efficacy.[136]
- Nicotine vaccines: Nicotine vaccines (e.g., NicVAX and TA-NIC) work by reducing the amount of nicotine reaching the brain, however, this method of therapy needs more investigations to establish its role and determine its side effects.[137]
- Technology and machine learning: Research studies using machine learning or artificial intelligence tools to provide feedback and communication with those who are trying to quit smoking are increasing, yet the findings are so far inconclusive.[138][139][140]
Special populations
Although smoking prevalence has declined in recent years leading up to 2016, certain subpopulations continue to smoke at disproportionately high rates and show resistance to cessation treatments.[141]
African Americans
The burden of tobacco-related disease such as tobacco-related cancers, heart disease and stroke is greater for African Americans than for European Americans.[142] Among ever smokers, 51% of European Americans but only 37% of African Americans had quit and among former smokers African Americans continued smoking for longer periods before quitting and age at quitting was older compared to European Americans.[143] Hence, the need for culturally-specific smoking cessation treatment.
Children and adolescents
Methods used with children and adolescents include:
- Motivational enhancement[144]
- Psychological support[144]
- Youth anti-tobacco activities, such as sport involvement
- School-based curricula, such as life-skills training
- School-based nurse counseling sessions[145]
- Access reduction to tobacco
- Anti-tobacco media[146][147]
- Family communication
Cochrane reviews, mainly of studies combining motivational enhancement and psychological support, concluded that "complex approaches" for smoking cessation among young people show promise.[144][148] The 2008 US Guideline recommends counselling-style support for adolescent smokers on the basis of a meta-analysis of seven studies.[13]:159–161 Neither the Cochrane review nor the 2008 Guideline recommends medications for adolescents who smoke.
Pregnant women
Smoking during pregnancy can cause adverse health effects in both the woman and the fetus. The 2008 US Guideline determined that "person-to-person psychosocial interventions" (typically including "intensive counseling") increased abstinence rates in pregnant women who smoke to 13.3%, compared with 7.6% in usual care.[13]:165–167 Mothers who smoke during pregnancy have a greater tendency towards premature births. Their babies are often underdeveloped, have smaller organs, and weigh much less than the normal baby. In addition, these babies have weaker immune systems, making them more susceptible to many diseases such as middle ear inflammations and asthmatic bronchitis as well as metabolic conditions such as diabetes and hypertension, all of which can bring significant morbidity.[149] Additionally, a study published by American Academy of Pediatrics shows that smoking during pregnancy increases the chance of sudden unexpected infant death (SUID).[150] There is also an increased chance that the child will be a smoker in adulthood. A systematic review showed that psychosocial interventions help women to stop smoking in late pregnancy and can reduce the incidence of low birthweight infants.[151]
It is a myth that a female smoker can cause harm to a fetus by quitting immediately upon discovering she is pregnant. This idea is not based on any medical study or fact.[152]
Schizophrenia
Studies across 20 countries show a strong association between patients with schizophrenia and smoking. People with schizophrenia are much more likely to smoke than those without the disease.[153] For example, in the United States, 80% or more of people with schizophrenia smoke, compared to 20% of the general population in 2006.[154]
Workers
A 2008 Cochrane review of smoking cessation activities in work-places concluded that "interventions directed towards individual smokers increase the likelihood of quitting smoking".[155] A 2010 systematic review determined that worksite incentives and competitions needed to be combined with additional interventions to produce significant increases in smoking cessation rates.[156]
Hospitalized smokers
Smokers who are hospitalised may be particularly motivated to quit.[13]:149–150 A 2012 Cochrane review found that interventions beginning during a hospital stay and continuing for one month or more after discharge were effective in producing abstinence.[158]
Patients undergoing elective surgery may get benefits of preoperative smoking cessation interventions, when starting 4–8 weeks before surgery with weekly counseling intervention for behavioral support and use of nicotine replacement therapy.[159] It is found to reduce the complications and the number of postoperative morbidity.[159]
Mood disorders
People who have mood disorders or attention deficit hyperactivity disorders have a greater chance to begin smoking and lower chance to quit smoking.[160] A higher correlation with smoking has also been seen in people diagnosed with major depressive disorder at any time throughout the duration of their lifetime as compared to those without the diagnosis. Success rates in quitting smoking were lower for those with a major depressive disorder diagnosis versus people without the diagnosis.[161] Exposure of cigarette smoke early on in life during pregnancy, infancy, or adolescence may negatively impact a child's neurodevelopment and increase the risk of developing anxiety disorders in the future.[162]
Homeless and poverty
Homelessness doubles the likelihood of an individual currently being a smoker. This is independent of other socioeconomic factors and behavioral health conditions. Homeless individuals have the same rates of the desire to quit smoking but are less likely than the general population to be successful in their attempt to quit.[163][164]
In the United States, 60-80% of homeless adults are current smokers. This is a considerably higher rate than that of the general adult population of 19%.[163] Many current smokers who are homeless report smoking as a means of coping with "all the pressure of being homeless."[163] The perception that homeless people smoking is "socially acceptable" can also reinforce these trends.[163]
Americans under the poverty line have higher rates of smoking and lower rates of quitting than those over the poverty line.[164][165][166] While the homeless population as a whole is concerned about short-term effects of smoking, such as shortness of breath of recurrent bronchitis, they are not as concerned with long-term consequences.[165] The homeless population has unique barriers to quit smoking such as unstructured days, the stress of finding a job, and immediate survival needs that supersede the desire to quit smoking.[165]
These unique barriers can be combated through pharmacotherapy and behavioral counseling for high levels of nicotine dependence, emphasis of immediate financial benefits to those who concern themselves with the short-term over the long-term, partnering with shelters to reduce the social acceptability of smoking in this population, and increased taxes on cigarettes and on alternative tobacco products to further make the addiction more difficult to fund.[167]
People living with HIV/AIDS
Smoking is very common among people living with HIV/AIDS and it impacts morbidity and mortality substantially. Combined smoking cessation interventions provide good long-term control.[168]
People in treatment for or recovery from substance use disorders
Over three-quarters of people in treatment for substance use are current smokers.[169] Providing counseling and pharmacotherapy (nicotine replacement therapy such as patches or gum, varenicline, and/or bupropion) increases tobacco abstinence without increasing the risk of returning to other substance use.[170]
Comparison of success rates
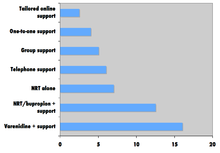
Comparison of success rates across interventions can be difficult because of different definitions of "success" across studies.[158] Robert West and Saul Shiffman, authorities in this field recognized by government health departments in a number of countries,[157]:73,76,80 have concluded that, used together, "behavioral support" and "medication" can quadruple the chances that a quit attempt will be successful.
A 2008 systematic review in the European Journal of Cancer Prevention found that group behavioural therapy was the most effective intervention strategy for smoking cessation, followed by bupropion, intensive physician advice, nicotine replacement therapy, individual counselling, telephone counselling, nursing interventions, and tailored self-help interventions; the study did not discuss varenicline.[172]
Factors affecting success
Quitting can be harder for individuals with dark pigmented skin compared to individuals with pale skin since nicotine has an affinity for melanin-containing tissues. Studies suggest this can cause the phenomenon of increased nicotine dependence and lower smoking cessation rate in darker pigmented individuals.[173]
There is an important social component to smoking. The spread of smoking cessation from person to person contributes to the decrease in smoking these years.[174] A 2008 study of a densely interconnected network of over 12,000 individuals found that smoking cessation by any given individual reduced the chances of others around them lighting up by the following amounts: a spouse by 67%, a sibling by 25%, a friend by 36%, and a coworker by 34%.[174] Nevertheless, a Cochrane review determined that interventions to increase social support for a smoker's cessation attempt did not increase long-term quit rates.[175]
Smokers who are trying to quit are faced with social influences that may persuade them to conform and continue smoking. Cravings are easier to detain when one's environment does not provoke the habit. If a person who stopped smoking has close relationships with active smokers, he or she is often put into situations that make the urge to conform more tempting. However, in a small group with at least one other not smoking, the likelihood of conformity decreases. The social influence to smoke cigarettes has been proven to rely on simple variables. One researched variable depends on whether the influence is from a friend or non-friend.[176] The research shows that individuals are 77% more likely to conform to non-friends, while close friendships decrease conformity. Therefore, if an acquaintance offers a cigarette as a polite gesture, the person who has stopped smoking will be more likely to break his commitment than if a friend had offered. Recent research from the International Tobacco Control (ITC) Four Country Survey of over 6,000 smokers found that smokers with fewer smoking friends were more likely to intend to quit and to succeed in their quit attempt.[177]
Expectations and attitude are significant factors. A self-perpetuating cycle occurs when a person feels bad for smoking yet smokes to alleviate feeling bad. Breaking that cycle can be a key in changing the sabotaging attitude.[178]
Smokers with major depressive disorder may be less successful at quitting smoking than non-depressed smokers.[13]:81[179]
Relapse (resuming smoking after quitting) has been related to psychological issues such as low self-efficacy,[180][181] or non-optimal coping responses;[182] however, psychological approaches to prevent relapse have not been proven to be successful.[183] In contrast, varenicline is suggested to have some effects and nicotine replacement therapy may help the unassisted abstainers.[183][184]
Side effects
| Craving for tobacco | 3 to 8 weeks[185] |
| Dizziness | Few days[185] |
| Insomnia | 1 to 2 weeks[185] |
| Headaches | 1 to 2 weeks[185] |
| Chest discomfort | 1 to 2 weeks[185] |
| Constipation | 1 to 2 weeks[185] |
| Irritability | 2 to 4 weeks[185] |
| Fatigue | 2 to 4 weeks[185] |
| Cough or nasal drip | Few weeks[185] |
| Lack of concentration | Few weeks[185] |
| Hunger | Up to several weeks[185] |
Symptoms
In a 2007 review of the effects of abstinence from tobacco, Hughes concluded that "anger, anxiety, depression, difficulty concentrating, impatience, insomnia, and restlessness are valid withdrawal symptoms that peak within the first week and last 2–4 weeks."[186] In contrast, "constipation, cough, dizziness, increased dreaming, and mouth ulcers" may or may not be symptoms of withdrawal, while drowsiness, fatigue, and certain physical symptoms ("dry mouth, flu symptoms, headaches, heart racing, skin rash, sweating, tremor") were not symptoms of withdrawal.[186]
Weight gain
Giving up smoking is associated with an average weight gain of 4–5 kilograms (8.8–11.0 lb) after 12 months, most of which occurs within the first three months of quitting.[187]
The possible causes of the weight gain include:
- Smoking over-expresses the gene AZGP1 which stimulates lipolysis, so smoking cessation may decrease lipolysis.[188]
- Smoking suppresses appetite, which may be caused by nicotine's effect on central autonomic neurons (e.g., via regulation of melanin concentrating hormone neurons in the hypothalamus).[189]
- Heavy smokers are reported to burn 200 calories per day more than non-smokers eating the same diet.[190] Possible reasons for this phenomenon include nicotine's ability to increase energy metabolism or nicotine's effect on peripheral neurons.[189]
The 2008 Guideline suggests that sustained-release bupropion, nicotine gum, and nicotine lozenge be used "to delay weight gain after quitting."[13]:173–176 A 2012 Cochrane review concluded that there is not sufficient evidence to recommend a particular program for preventing weight gain.[191]
Depression
Like other physically addictive drugs, nicotine addiction causes a down-regulation of the production of dopamine and other stimulatory neurotransmitters as the brain attempts to compensate for the artificial stimulation caused by smoking. Therefore, when people stop smoking, depressive symptoms such as suicidal tendencies or actual depression may result,[179][192] although a recent international study comparing smokers who had stopped for 3 months with continuing smokers found that stopping smoking did not appear to increase anxiety or depression.[193] This side effect of smoking cessation may be particularly common in women, as depression is more common among women than among men.[194]
Anxiety
A recent study by The British Journal of Psychiatry has found that smokers who successfully quit feel less anxious afterward with the effect being greater among those who had mood and anxiety disorders than those that smoked for pleasure.[195]
Health benefits
Many of tobacco's detrimental health effects can be reduced or largely removed through smoking cessation. The health benefits over time of stopping smoking include:[197]
- Within 20 minutes after quitting, blood pressure and heart rate decrease
- Within 12 hours, carbon monoxide levels in the blood decrease to normal
- Within 48 hours, nerve endings and sense of smell and taste both start recovering
- Within 3 months, circulation and lung function improve
- Within 9 months, there are decreases in cough and shortness of breath
- Within 1 year, the risk of coronary heart disease is cut in half
- Within 5 years, the risk of stroke falls to the same as a non-smoker, and the risks of many cancers (mouth, throat, esophagus, bladder, cervix) decrease significantly
- Within 10 years, the risk of dying from lung cancer is cut in half,[198] and the risks of larynx and pancreas cancers decrease
- Within 15 years, the risk of coronary heart disease drops to the level of a non-smoker; lowered risk for developing COPD (chronic obstructive pulmonary disease)
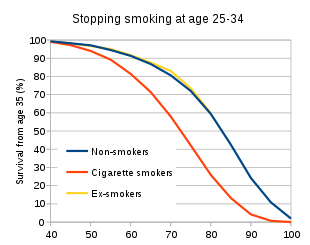
The British Doctors Study showed that those who stopped smoking before they reached 30 years of age lived almost as long as those who never smoked.[196] Stopping in one's sixties can still add three years of healthy life.[196] A randomized trial from the U.S. and Canada showed that a smoking cessation program lasting 10 weeks decreased mortality from all causes over 14 years later.[199] A recent article on mortality in a cohort of 8,645 smokers who were followed-up after 43 years determined that “current smoking and lifetime persistent smoking were associated with an increased risk of all-cause, CVD [cardiovascular disease], COPD [chronic obstructive pulmonary disease], and any cancer, and lung cancer mortality.[200]
Another published study, "Smoking Cessation Reduces Postoperative Complications: A Systematic Review and Meta-analysis", examined six randomized trials and 15 observational studies to look at the effects of preoperative smoking cessation on postoperative complications. The findings were: 1) taken together, the studies demonstrated decreased the likelihood of postoperative complications in patients who ceased smoking prior to surgery; 2) overall, each week of cessation prior to surgery increased the magnitude of the effect by 19%. A significant positive effect was noted in trials where smoking cessation occurred at least four weeks prior to surgery; 3) For the six randomized trials, they demonstrated on average a relative risk reduction of 41% for postoperative complications.[201]
Cost-effectiveness
Cost-effectiveness analyses of smoking cessation activities have shown that they increase quality-adjusted life years (QALYs) at costs comparable with other types of interventions to treat and prevent disease.[13]:134–137 Studies of the cost-effectiveness of smoking cessation include:
- In a 1997 U.S. analysis, the estimated cost per QALY varied by the type of cessation approach, ranging from group intensive counselling without nicotine replacement at $1108 per QALY to minimal counselling with nicotine gum at $4542 per QALY.[202]
- A study from Erasmus University Rotterdam limited to people with chronic obstructive pulmonary disease found that the cost-effectiveness of minimal counselling, intensive counselling, and drug therapy were €16,900, €8,200, and €2,400 per QALY gained respectively.[203]
- Among National Health Service smoking cessation clients in Glasgow, pharmacy one-to-one counselling cost £2,600 per QALY gained and group support cost £4,800 per QALY gained.[204]
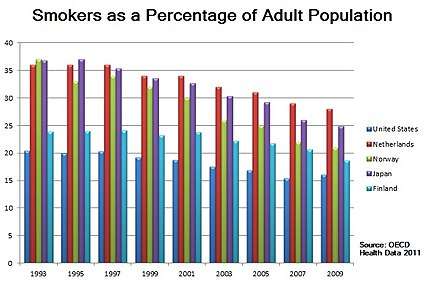
Statistical trends
The frequency of smoking cessation among smokers varies across countries. Smoking cessation increased in Spain between 1965 and 2000,[205] in Scotland between 1998 and 2007,[206] and in Italy after 2000.[207] In contrast, in the U.S. the cessation rate was "stable (or varied little)" between 1998 and 2008,[208] and in China smoking cessation rates declined between 1998 and 2003.[209]
Nevertheless, in a growing number of countries there are now more ex-smokers than smokers [23] For example, in the U.S. as of 2010, there were 47 million ex-smokers and 46 million smokers.[210] In 2014, the Centers for Disease Control and Prevention reports that the number of adult smokers, 18 years and older, in the U.S. has fallen to 40 million current smokers.[211]
See also
- Breath carbon monoxide monitor
- Coerced abstinence
- Health effects of tobacco
- Health promotion
- Massachusetts Tobacco Cessation and Prevention Program
- Nicotine Anonymous
- Nicotine replacement therapy
- Smoking cessation programs in Canada
- Tobacco cessation clinics in India
- Tobacco control
- Tobacco Control (journal)
- Truth Initiative (formerly American Legacy Foundation)
- U.S. government and smoking cessation
- Youth Tobacco Cessation Collaborative
- World No Tobacco Day
Bibliography
- "Take steps NOW to stop smoking". nhs.uk. 27 April 2018.
- "How to Quit Smoking or Smokeless Tobacco". American Cancer Society. 2016. Retrieved 2016-07-22.
- Mooney ME, Johnson EO, Breslau N, Bierut LJ, Hatsukami DK (June 2011). "Cigarette smoking reduction and changes in nicotine dependence". Nicotine & Tobacco Research. 13 (6): 426–30. doi:10.1093/ntr/ntr019. PMC 3103717. PMID 21367813.
- Sudeep CB (2017). "Tobacco Cessation Counseling – the Roles and Responsibilities of a Dentist". International Journal of Advanced Research. 5 (3): 326–331. doi:10.21474/IJAR01/3518.
- Centers for Disease Control Prevention (CDC) (November 2011). "Quitting smoking among adults--United States, 2001-2010". MMWR. Morbidity and Mortality Weekly Report. 60 (44): 1513–9. PMID 22071589. Retrieved 2015-05-09.
- Temitayo Orisasami I, Ojo O (July 2016). "Evaluating the effectiveness of smoking cessation in the management of COPD". British Journal of Nursing. 25 (14): 786–91. doi:10.12968/bjon.2016.25.14.786. PMID 27467642.
- "WHO Report on the global tobacco epidemic". World Health Organization. 2015.
- Rigotti NA (October 2012). "Strategies to help a smoker who is struggling to quit". JAMA. 308 (15): 1573–80. doi:10.1001/jama.2012.13043. PMC 4562427. PMID 23073954.
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T (March 2016). "Combined pharmacotherapy and behavioural interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 3: CD008286. doi:10.1002/14651858.CD008286.pub3. PMID 27009521.
- Rosen LJ, Galili T, Kott J, Goodman M, Freedman LS (May 2018). "Diminishing benefit of smoking cessation medications during the first year: a meta-analysis of randomized controlled trials". Addiction. 113 (5): 805–816. doi:10.1111/add.14134. PMC 5947828. PMID 29377409.
- Benowitz NL (June 2010). "Nicotine addiction". The New England Journal of Medicine. 362 (24): 2295–303. doi:10.1056/NEJMra0809890. PMC 2928221. PMID 20554984.
- Cochrane Tobacco Addiction Group (2016). "Cochrane Tobacco Addiction". Retrieved 2016-07-06.
- Fiore MC, Jaén CR, Baker TB (2008). Clinical practice guideline: treating tobacco use and dependence: 2008 update (PDF). Rockville, MD: U.S. Department of Health and Human Services, Public Health Service. Archived from the original on 2016-03-27. Retrieved 2016-07-06.CS1 maint: unfit url (link)
- Fiore MC, Bailey WC, Cohen SJ (1996). Smoking cessation. Clinical practice guideline no. 18. AHCPR publication no. 96-0692. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research.
- Fiore MC, Bailey WC, Cohen SJ (2000). Clinical practice guideline: treating tobacco use and dependence (PDF). Rockville, MD: U.S. Department of Health and Human Services, Public Health Service. Archived from the original on 2011-01-07. Retrieved 2011-02-16.CS1 maint: unfit url (link)
- Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, Schwartz R (June 2016). "Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers". BMJ Open. 6 (6): e011045. doi:10.1136/bmjopen-2016-011045. PMC 4908897. PMID 27288378.
- Caraballo RS, Shafer PR, Patel D, Davis KC, McAfee TA (April 2017). "Quit Methods Used by US Adult Cigarette Smokers, 2014-2016". Preventing Chronic Disease. 14: E32. doi:10.5888/pcd14.160600. PMC 5392446. PMID 28409740.
- Hughes JR, Keely J, Naud S (January 2004). "Shape of the relapse curve and long-term abstinence among untreated smokers". Addiction. 99 (1): 29–38. doi:10.1111/j.1360-0443.2004.00540.x. PMID 14678060.
- Edwards SA, Bondy SJ, Callaghan RC, Mann RE (March 2014). "Prevalence of unassisted quit attempts in population-based studies: a systematic review of the literature". Addictive Behaviors. 39 (3): 512–9. doi:10.1016/j.addbeh.2013.10.036. PMID 24333037.
- Lee CW, Kahende J (August 2007). "Factors associated with successful smoking cessation in the United States, 2000". American Journal of Public Health. 97 (8): 1503–9. doi:10.2105/AJPH.2005.083527. PMC 1931453. PMID 17600268.
- Fiore MC, Novotny TE, Pierce JP, Giovino GA, Hatziandreu EJ, Newcomb PA, Surawicz TS, Davis RM (1990). "Methods used to quit smoking in the United States. Do cessation programs help?". JAMA. 263 (20): 2760–5. doi:10.1001/jama.1990.03440200064024. PMID 2271019.
- Doran CM, Valenti L, Robinson M, Britt H, Mattick RP (May 2006). "Smoking status of Australian general practice patients and their attempts to quit". Addictive Behaviors. 31 (5): 758–66. doi:10.1016/j.addbeh.2005.05.054. PMID 16137834.
- Chapman S, MacKenzie R (February 2010). "The global research neglect of unassisted smoking cessation: causes and consequences". PLOS Medicine. 7 (2): e1000216. doi:10.1371/journal.pmed.1000216. PMC 2817714. PMID 20161722.
- Hung WT, Dunlop SM, Perez D, Cotter T (July 2011). "Use and perceived helpfulness of smoking cessation methods: results from a population survey of recent quitters". BMC Public Health. 11: 592. doi:10.1186/1471-2458-11-592. PMC 3160379. PMID 21791111.
- "Guide to quitting smoking. What do I need to know about quitting" (PDF). American Cancer Society. 2014.
- Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T (May 2018). "Nicotine replacement therapy versus control for smoking cessation". The Cochrane Database of Systematic Reviews. 5: CD000146. doi:10.1002/14651858.CD000146.pub5. PMC 6353172. PMID 29852054.
- Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T (May 2018). "Nicotine replacement therapy versus control for smoking cessation". The Cochrane Database of Systematic Reviews. 5 (5): CD000146. doi:10.1002/14651858.CD000146.pub5. PMC 6353172. PMID 29852054.
- Zhou HX (November 2008). "The debut of PMC Biophysics". PMC Biophysics. 1 (1): 1. doi:10.1186/1757-5036-1-1. PMC 2605105. PMID 19351423.
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML (2005). "Pharmacotherapy for nicotine dependence". Ca. 55 (5): 281–99, quiz 322–3, 325. doi:10.3322/canjclin.55.5.281. PMID 16166074.
- Millstone K (2007-02-13). "Nixing the patch: Smokers quit cold turkey". Columbia.edu News Service. Archived from the original on 2018-12-25. Retrieved 2011-02-21.CS1 maint: unfit url (link)
- Lancaster T, Stead LF (2000). "Mecamylamine (a nicotine antagonist) for smoking cessation". The Cochrane Database of Systematic Reviews (2): CD001009. doi:10.1002/14651858.CD001009. PMC 7271835. PMID 10796584.
- Howes S, Hartmann-Boyce J, Livingstone-Banks J, Hong B, Lindson N (22 April 2020). "Antidepressants for smoking cessation". The Cochrane Database of Systematic Reviews (4): CD000031. doi:10.1002/14651858.CD000031.pub5. PMC 7175455. PMID 32319681.
- Pfizer (2015). "Product monograph Champix" (PDF). Archived from the original on 2015-11-16.CS1 maint: unfit url (link)
- Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T (May 2016). "Nicotine receptor partial agonists for smoking cessation". The Cochrane Database of Systematic Reviews (5): CD006103. doi:10.1002/14651858.CD006103.pub7. hdl:1983/0cbd99fb-032e-4547-95cc-e314a8d042d7. PMC 6464943. PMID 27158893.
- Sterling LH, Windle SB, Filion KB, Touma L, Eisenberg MJ (February 2016). "Varenicline and Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". Journal of the American Heart Association. 5 (2): e002849. doi:10.1161/JAHA.115.002849. PMC 4802486. PMID 26903004.
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE (June 2016). "Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial". Lancet. 387 (10037): 2507–20. doi:10.1016/s0140-6736(16)30272-0. PMID 27116918.
- Gourlay SG, Stead LF, Benowitz NL (2004). "Clonidine for smoking cessation". The Cochrane Database of Systematic Reviews (3): CD000058. doi:10.1002/14651858.CD000058.pub2. PMC 7038651. PMID 15266422.
- Hughes JR, Stead LF, Lancaster T (2000). "Anxiolytics for smoking cessation". The Cochrane Database of Systematic Reviews (4): CD002849. doi:10.1002/14651858.CD002849. PMID 11034774.
- Cahill K, Ussher MH (March 2011). "Cannabinoid type 1 receptor antagonists for smoking cessation". The Cochrane Database of Systematic Reviews (3): CD005353. doi:10.1002/14651858.CD005353.pub4. PMC 6486173. PMID 21412887.
- Phend C (2009-04-03). "Gradual cutback with nicotine replacement boosts quit rates". MedPage Today. Retrieved 2011-02-20.
- Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P (April 2009). "Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis". BMJ. 338: b1024. doi:10.1136/bmj.b1024. PMC 2664870. PMID 19342408.
- Lindson N, Klemperer E, Hong B, Ordóñez-Mena JM, Aveyard P (September 2019). "Smoking reduction interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 9: CD013183. doi:10.1002/14651858.CD013183.pub2. PMC 6953262. PMID 31565800.
- "Making a Quit Plan". Centers for Disease Control and Prevention. Retrieved 19 October 2015.
- "Preparing for Quit Day". Mayo Clinic. Mayo Clinic. Retrieved 19 October 2015.
- Smit ES, Hoving C, Schelleman-Offermans K, West R, de Vries H (September 2014). "Predictors of successful and unsuccessful quit attempts among smokers motivated to quit" (PDF). Addictive Behaviors. 39 (9): 1318–24. doi:10.1016/j.addbeh.2014.04.017. PMID 24837754.
- de Vries H, Eggers SM, Bolman C (April 2013). "The role of action planning and plan enactment for smoking cessation". BMC Public Health. 13: 393. doi:10.1186/1471-2458-13-393. PMC 3644281. PMID 23622256.
- Bolman C, Eggers SM, van Osch L, Te Poel F, Candel M, de Vries H (Oct 2015). "Is Action Planning Helpful for Smoking Cessation? Assessing the Effects of Action Planning in a Web-Based Computer-Tailored Intervention". Substance Use & Misuse. 50 (10): 1249–60. doi:10.3109/10826084.2014.977397. PMID 26440754.
- Ayers JW, Althouse BM, Johnson M, Cohen JE (January 2014). "Circaseptan (weekly) rhythms in smoking cessation considerations". JAMA Internal Medicine. 174 (1): 146–8. doi:10.1001/jamainternmed.2013.11933. PMC 4670616. PMID 24166181.
- Erbas B, Bui Q, Huggins R, Harper T, White V (February 2006). "Investigating the relation between placement of Quit antismoking advertisements and number of telephone calls to Quitline: a semiparametric modelling approach". Journal of Epidemiology and Community Health. 60 (2): 180–2. doi:10.1136/jech.2005.038109. PMC 2566152. PMID 16415271.
- Secker-Walker RH, Gnich W, Platt S, Lancaster T (2002). "Community interventions for reducing smoking among adults". The Cochrane Database of Systematic Reviews (3): CD001745. doi:10.1002/14651858.CD001745. PMC 6464950. PMID 12137631.
- Lemmens V, Oenema A, Knut IK, Brug J (November 2008). "Effectiveness of smoking cessation interventions among adults: a systematic review of reviews" (PDF). European Journal of Cancer Prevention. 17 (6): 535–44. doi:10.1097/CEJ.0b013e3282f75e48. PMID 18941375. Archived from the original (PDF) on 2011-07-06.
- "State-Mandated Tobacco Ban, Integration of Cessation Services, and Other Policies Reduce Smoking Among Patients and Staff at Substance Abuse Treatment Centers". Agency for Healthcare Research and Quality. 2013-02-27. Retrieved 2013-05-13.
- Centers for Disease Control and Prevention (CDC) (May 2007). "State-specific prevalence of smoke-free home rules--United States, 1992-2003". MMWR. Morbidity and Mortality Weekly Report. 56 (20): 501–4. PMID 17522588.
- King BA, Dube SR, Homa DM (May 2013). "Smoke-free rules and secondhand smoke exposure in homes and vehicles among US adults, 2009-2010". Preventing Chronic Disease. 10: E79. doi:10.5888/pcd10.120218. PMC 3666976. PMID 23680508.
- King BA, Babb SD, Tynan MA, Gerzoff RB (July 2013). "National and state estimates of secondhand smoke infiltration among U.S. multiunit housing residents". Nicotine & Tobacco Research. 15 (7): 1316–21. doi:10.1093/ntr/nts254. PMC 4571449. PMID 23248030.
- Hopkins DP, Briss PA, Ricard CJ, Husten CG, Carande-Kulis VG, Fielding JE, Alao MO, McKenna JW, Sharp DJ, Harris JR, Woollery TA, Harris KW (February 2001). "Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke" (PDF). American Journal of Preventive Medicine. 20 (2 Suppl): 16–66. doi:10.1016/S0749-3797(00)00297-X. PMID 11173215.
- Bala MM, Strzeszynski L, Topor-Madry R (November 2017). "Mass media interventions for smoking cessation in adults". The Cochrane Database of Systematic Reviews. 11: CD004704. doi:10.1002/14651858.CD004704.pub4. PMC 6486126. PMID 29159862.
- Frazer K, McHugh J, Callinan JE, Kelleher C (May 2016). "Impact of institutional smoking bans on reducing harms and secondhand smoke exposure". The Cochrane Database of Systematic Reviews (5): CD011856. doi:10.1002/14651858.CD011856.pub2. PMID 27230795.
- Zhu SH, Anderson CM, Tedeschi GJ, Rosbrook B, Johnson CE, Byrd M, Gutiérrez-Terrell E (October 2002). "Evidence of real-world effectiveness of a telephone quitline for smokers" (PDF). The New England Journal of Medicine. 347 (14): 1087–93. doi:10.1056/NEJMsa020660. PMID 12362011.
- Helgason AR, Tomson T, Lund KE, Galanti R, Ahnve S, Gilljam H (September 2004). "Factors related to abstinence in a telephone helpline for smoking cessation". European Journal of Public Health. 14 (3): 306–10. doi:10.1093/eurpub/14.3.306. PMID 15369039.
- Lancaster T, Stead LF (March 2017). "Individual behavioural counselling for smoking cessation". The Cochrane Database of Systematic Reviews. 3: CD001292. doi:10.1002/14651858.CD001292.pub3. PMC 6464359. PMID 28361496.
- Matkin W, Ordóñez-Mena JM, Hartmann-Boyce J (May 2019). Cochrane Tobacco Addiction Group (ed.). "Telephone counselling for smoking cessation". The Cochrane Database of Systematic Reviews. 5: CD002850. doi:10.1002/14651858.CD002850.pub4. PMC 6496404. PMID 31045250.
- Hartmann-Boyce, Jamie; Hong, Bosun; Livingstone-Banks, Jonathan; Wheat, Hannah; Fanshawe, Thomas R. (5 June 2019). "Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation". The Cochrane Database of Systematic Reviews. 6: CD009670. doi:10.1002/14651858.CD009670.pub4. ISSN 1469-493X. PMC 6549450. PMID 31166007.
- Baskerville NB, Azagba S, Norman C, McKeown K, Brown KS (March 2016). "Effect of a Digital Social Media Campaign on Young Adult Smoking Cessation". Nicotine & Tobacco Research. 18 (3): 351–60. doi:10.1093/ntr/ntv119. PMID 26045252.
- Stead LF, Carroll AJ, Lancaster T (March 2017). "Group behaviour therapy programmes for smoking cessation". The Cochrane Database of Systematic Reviews. 3: CD001007. doi:10.1002/14651858.CD001007.pub3. PMC 6464070. PMID 28361497.
- Stead, Lindsay F.; Carroll, Allison J.; Lancaster, Tim (31 March 2017). "Group behaviour therapy programmes for smoking cessation". The Cochrane Database of Systematic Reviews. 3: CD001007. doi:10.1002/14651858.CD001007.pub3. ISSN 1469-493X. PMC 6464070. PMID 28361497.
- Lancaster, Tim; Stead, Lindsay F. (31 March 2017). "Individual behavioural counselling for smoking cessation". The Cochrane Database of Systematic Reviews. 3: CD001292. doi:10.1002/14651858.CD001292.pub3. ISSN 1469-493X. PMC 6464359. PMID 28361496.
- Lindson-Hawley N, Thompson TP, Begh R (March 2015). "Motivational interviewing for smoking cessation". The Cochrane Database of Systematic Reviews (3): CD006936. doi:10.1002/14651858.CD006936.pub3. PMID 25726920.
- Hettema JE, Hendricks PS (December 2010). "Motivational interviewing for smoking cessation: a meta-analytic review". Journal of Consulting and Clinical Psychology. 78 (6): 868–84. doi:10.1037/a0021498. PMID 21114344.
- Heckman CJ, Egleston BL, Hofmann MT (October 2010). "Efficacy of motivational interviewing for smoking cessation: a systematic review and meta-analysis". Tobacco Control. 19 (5): 410–6. doi:10.1136/tc.2009.033175. PMC 2947553. PMID 20675688.
- Perkins KA, Conklin CA, Levine MD (2008). Cognitive-behavioral therapy for smoking cessation: a practical guidebook to the most effective treatment. New York: Routledge. ISBN 978-0-415-95463-1.
- Ruiz FJ (2010). "A review of Acceptance and Commitment Therapy (ACT) empirical evidence: Correlational, experimental psychopathology, component and outcome studies". International Journal of Psychology and Psychological Therapy. 10 (1): 125–62.
- "About Freedom From Smoking". American Lung Association.
- Prochaska JO, Velicer WF, DiClemente CC, Fava J (August 1988). "Measuring processes of change: applications to the cessation of smoking". Journal of Consulting and Clinical Psychology. 56 (4): 520–8. doi:10.1037/0022-006X.56.4.520. PMID 3198809.
- DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS (April 1991). "The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change" (PDF). Journal of Consulting and Clinical Psychology. 59 (2): 295–304. doi:10.1037/0022-006X.59.2.295. PMID 2030191. Archived from the original on 2011-06-06.CS1 maint: unfit url (link)
- Velicer WF, Prochaska JO, Rossi JS, Snow MG (January 1992). "Assessing outcome in smoking cessation studies". Psychological Bulletin. 111 (1): 23–41. doi:10.1037/0033-2909.111.1.23. PMID 1539088.
- Prochaska JO, DiClemente CC, Velicer WF, Rossi JS (September 1993). "Standardized, individualized, interactive, and personalized self-help programs for smoking cessation" (PDF). Health Psychology. 12 (5): 399–405. doi:10.1037/0278-6133.12.5.399. PMID 8223364. Archived from the original on 2011-06-06.CS1 maint: unfit url (link)
- Cahill K, Lancaster T, Green N (November 2010). Cahill K (ed.). "Stage-based interventions for smoking cessation". The Cochrane Database of Systematic Reviews (11): CD004492. doi:10.1002/14651858.CD004492.pub4. PMID 21069681.
- Livingstone-Banks J, Ordóñez-Mena JM, Hartmann-Boyce J (January 2019). "Print-based self-help interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 1: CD001118. doi:10.1002/14651858.CD001118.pub4. PMC 7112723. PMID 30623970.
- "Nicotine Anonymous offers help for those who desire to live free from nicotine". nicotine-anonymous.org.
- Glasser I (February 2010). "Nicotine anonymous may benefit nicotine-dependent individuals". American Journal of Public Health. 100 (2): 196, author reply 196–7. doi:10.2105/ajph.2009.181545. PMC 2804638. PMID 20019295.
- U.S. Department of Health and Human Services. "MySmokeFree: Your personalized quit experience". Smokefree.gov.
- "Slideshow: 13 Best Quit-Smoking Tips Ever". WebMD.
- Hendrick B. "Computer is an ally in quit-smoking fight". WebMD.
- Myung SK, McDonnell DD, Kazinets G, Seo HG, Moskowitz JM (May 2009). "Effects of Web- and computer-based smoking cessation programs: meta-analysis of randomized controlled trials". Archives of Internal Medicine. 169 (10): 929–37. doi:10.1001/archinternmed.2009.109. PMID 19468084.
- Taylor GM, Dalili MN, Semwal M, Civljak M, Sheikh A, Car J (September 2017). "Internet-based interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 9: CD007078. doi:10.1002/14651858.CD007078.pub5. PMC 6703145. PMID 28869775.
- Hutton HE, Wilson LM, Apelberg BJ, Tang EA, Odelola O, Bass EB, Chander G (April 2011). "A systematic review of randomized controlled trials: Web-based interventions for smoking cessation among adolescents, college students, and adults". Nicotine & Tobacco Research. 13 (4): 227–38. doi:10.1093/ntr/ntq252. PMID 21350042.
- Whittaker R, McRobbie H, Bullen C, Rodgers A, Gu Y (April 2016). "Mobile phone-based interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 4: CD006611. doi:10.1002/14651858.CD006611.pub4. PMC 6485940. PMID 27060875.
- Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, Rodgers A, Cairns J, Kenward MG, Roberts I (July 2011). "Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial". Lancet. 378 (9785): 49–55. doi:10.1016/s0140-6736(11)60701-0. PMC 3143315. PMID 21722952.
- Free C, Phillips G, Watson L, Galli L, Felix L, Edwards P, Patel V, Haines A (2013). "The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis". PLOS Medicine. 10 (1): e1001363. doi:10.1371/journal.pmed.1001363. PMC 3566926. PMID 23458994.
- Brendryen H, Kraft P (March 2008). "Happy ending: a randomized controlled trial of a digital multi-media smoking cessation intervention". Addiction. 103 (3): 478–84, discussion 485–6. doi:10.1111/j.1360-0443.2007.02119.x. PMID 18269367.
- Brendryen H, Drozd F, Kraft P (November 2008). "A digital smoking cessation program delivered through internet and cell phone without nicotine replacement (happy ending): randomized controlled trial". Journal of Medical Internet Research. 10 (5): e51. doi:10.2196/jmir.1005. PMC 2630841. PMID 19087949.
- Carr A (2004). The easy way to stop smoking. New York: Sterling. ISBN 978-1-4027-7163-7.
- Gonzales D, Redtomahawk D, Pizacani B, Bjornson WG, Spradley J, Allen E, Lees P (February 2007). "Support for spirituality in smoking cessation: results of pilot survey". Nicotine & Tobacco Research. 9 (2): 299–303. doi:10.1080/14622200601078582. PMID 17365761.
- Tang YY, Tang R, Posner MI (June 2016). "Mindfulness meditation improves emotion regulation and reduces drug abuse". Drug and Alcohol Dependence. 163 Suppl 1: S13-8. doi:10.1016/j.drugalcdep.2015.11.041. PMID 27306725.
- Ussher, Michael H.; Faulkner, Guy E. J.; Angus, Kathryn; Hartmann-Boyce, Jamie; Taylor, Adrian H. (30 October 2019). "Exercise interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 2019 (10). doi:10.1002/14651858.CD002295.pub6. ISSN 1469-493X. PMC 6819982. PMID 31684691.
- Bittoun R (2008). "Carbon monoxide meter: The essential clinical tool- the 'stethoscope"-of smoking cessation". Journal of Smoking Cessation. 3 (2): 69–70. doi:10.1375/jsc.3.2.69.
- Jamrozik K, Vessey M, Fowler G, Wald N, Parker G, Van Vunakis H (May 1984). "Controlled trial of three different antismoking interventions in general practice". British Medical Journal. 288 (6429): 1499–503. doi:10.1136/bmj.288.6429.1499. PMC 1441184. PMID 6426618.
- Clair C, Mueller Y, Livingstone-Banks J, Burnand B, Camain JY, Cornuz J, Rège-Walther M, Selby K, Bize R (March 2019). "Biomedical risk assessment as an aid for smoking cessation". The Cochrane Database of Systematic Reviews. 3: CD004705. doi:10.1002/14651858.CD004705.pub5. PMC 6434771. PMID 30912847.
- Irving JM, Clark EC, Crombie IK, Smith WC (January 1988). "Evaluation of a portable measure of expired-air carbon monoxide". Preventive Medicine. 17 (1): 109–15. doi:10.1016/0091-7435(88)90076-x. PMID 3362796.
- Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G (February 2009). "Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology". Therapeutic Drug Monitoring. 31 (1): 14–30. doi:10.1097/FTD.0b013e3181957a3b. PMC 3644554. PMID 19125149.
- McClure JB (2001). "Are biomarkers a useful aid in smoking cessation? A review and analysis of the literature". Behavioral Medicine. 27 (1): 37–47. doi:10.1080/08964280109595770. PMID 11575171.
- Notley, Caitlin; Gentry, Sarah; Livingstone-Banks, Jonathan; Bauld, Linda; Perera, Rafael; Hartmann-Boyce, Jamie (17 July 2019). "Incentives for smoking cessation". The Cochrane Database of Systematic Reviews. 7: CD004307. doi:10.1002/14651858.CD004307.pub6. ISSN 1469-493X. PMC 6635501. PMID 31313293.
- Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, Loewenstein G, Brennan TA, Asch DA, Volpp KG (May 2015). "Randomized trial of four financial-incentive programs for smoking cessation". The New England Journal of Medicine. 372 (22): 2108–17. doi:10.1056/NEJMoa1414293. PMC 4471993. PMID 25970009.
- Fanshawe, Thomas R; Hartmann-Boyce, Jamie; Perera, Rafael; Lindson, Nicola (2019). "Competitions for smoking cessation". Cochrane Database of Systematic Reviews. 2: CD013272. doi:10.1002/14651858.CD013272. ISSN 1465-1858. PMC 6953205. PMID 30784046.
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T (May 2013). "Physician advice for smoking cessation". The Cochrane Database of Systematic Reviews (5): CD000165. doi:10.1002/14651858.CD000165.pub4. PMC 7064045. PMID 23728631.
- Maguire CP, Ryan J, Kelly A, O'Neill D, Coakley D, Walsh JB (May 2000). "Do patient age and medical condition influence medical advice to stop smoking?". Age and Ageing. 29 (3): 264–6. doi:10.1093/ageing/29.3.264. PMID 10855911.
- Ossip-Klein DJ, McIntosh S, Utman C, Burton K, Spada J, Guido J (October 2000). "Smokers ages 50+: who gets physician advice to quit?". Preventive Medicine. 31 (4): 364–9. doi:10.1006/pmed.2000.0721. PMID 11006061.
- Rice VH, Heath L, Livingstone-Banks J, Hartmann-Boyce J (December 2017). "Nursing interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 12: CD001188. doi:10.1002/14651858.CD001188.pub5. PMC 6486227. PMID 29243221.
- Carson-Chahhoud, Kristin V.; Livingstone-Banks, Jonathan; Sharrad, Kelsey J.; Kopsaftis, Zoe; Brinn, Malcolm P.; To-A-Nan, Rachada; Bond, Christine M. (31 October 2019). "Community pharmacy personnel interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 2019 (10). doi:10.1002/14651858.CD003698.pub3. ISSN 1469-493X. PMC 6822095. PMID 31684695.
- Carr AB, Ebbert J. interventions for tobacco cessation in the dental setting. Cochrane Database of Systematic Reviews 2012, Issue 6. Art. No.: CD005084. doi:10.1002/14651858.CD005084.pub3
- Carson KV, Verbiest ME, Crone MR, Brinn MP, Esterman AJ, Assendelft WJ, Smith BJ (May 2012). Carson KV (ed.). "Training health professionals in smoking cessation" (PDF). The Cochrane Database of Systematic Reviews. 5 (5): CD000214. doi:10.1002/14651858.CD000214.pub2. PMID 22592671.
- Reda, Ayalu A.; Kotz, Daniel; Evers, Silvia M. A. A.; van Schayck, Constant Paul (2012-06-13). Van Schayck, Constant Paul (ed.). "Healthcare financing systems for increasing the use of tobacco dependence treatment". The Cochrane Database of Systematic Reviews (6): CD004305. doi:10.1002/14651858.CD004305.pub4. ISSN 1469-493X. PMID 22696341.
- Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A (2010). "Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis". Preventive Medicine. 51 (3–4): 199–213. doi:10.1016/j.ypmed.2010.06.007. PMID 20600264.
- Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T, et al. (Cochrane Tobacco Addiction Group) (May 2018). "Nicotine replacement therapy versus control for smoking cessation". The Cochrane Database of Systematic Reviews. 5: CD000146. doi:10.1002/14651858.CD000146.pub5. PMC 6353172. PMID 29852054.
- McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P (2014). McRobbie H (ed.). "Electronic cigarettes for smoking cessation and reduction". The Cochrane Database of Systematic Reviews. 12 (12): CD010216. doi:10.1002/14651858.CD010216.pub2. PMID 25515689.
- Camenga DR, Tindle HA (July 2018). "Weighing the Risks and Benefits of Electronic Cigarette Use in High-Risk Populations". The Medical Clinics of North America. 102 (4): 765–779. doi:10.1016/j.mcna.2018.03.002. PMID 29933828.
- Formanek P, Salisbury-Afshar E, Afshar M (August 2018). "Helping Patients With ESRD and Earlier Stages of CKD to Quit Smoking". American Journal of Kidney Diseases. 72 (2): 255–266. doi:10.1053/j.ajkd.2018.01.057. PMC 6057817. PMID 29661542.
- Royal College of Physicians (25 June 2014). "RCP statement on e-cigarettes". RCP London.
- McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H (August 2015). "E-cigarettes: an evidence update" (PDF). UK: Public Health England. p. 6.
- He D, Berg JE, Høstmark AT (March 1997). "Effects of acupuncture on smoking cessation or reduction for motivated smokers". Preventive Medicine. 26 (2): 208–14. doi:10.1006/pmed.1996.0125. PMID 9085389.
- White AR, Rampes H, Liu JP, Stead LF, Campbell J (January 2014). "Acupuncture and related interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 1 (1): CD000009. doi:10.1002/14651858.CD000009.pub4. PMC 7263424. PMID 24459016.
- US National Library of Medicine. "Smoking - tips on how to quit: MedlinePlus Medical Encyclopedia". medlineplus.gov.
- "Hypnosis for Quitting Smoking". WebMD. Retrieved 19 May 2012.
- Barnes, Joanne; McRobbie, Hayden; Dong, Christine Y.; Walker, Natalie; Hartmann-Boyce, Jamie (14 June 2019). "Hypnotherapy for smoking cessation". The Cochrane Database of Systematic Reviews. 6: CD001008. doi:10.1002/14651858.CD001008.pub3. ISSN 1469-493X. PMC 6568235. PMID 31198991.
- Johnson DL, Karkut RT (October 1994). "Performance by gender in a stop-smoking program combining hypnosis and aversion". Psychological Reports. 75 (2): 851–7. doi:10.2466/pr0.1994.75.2.851. PMID 7862796.
- Law M, Tang JL (October 1995). "An analysis of the effectiveness of interventions intended to help people stop smoking". Archives of Internal Medicine. 155 (18): 1933–41. doi:10.1001/archinte.1995.00430180025004. PMID 7575046.
- Barnes J, McRobbie H, Dong CY, Walker N, Hartmann-Boyce J (June 2019). "Hypnotherapy for smoking cessation". The Cochrane Database of Systematic Reviews. 6: CD001008. doi:10.1002/14651858.CD001008.pub3. PMC 6568235. PMID 31198991.
- Carmody TP, Duncan C, Simon JA, Solkowitz S, Huggins J, Lee S, Delucchi K (May 2008). "Hypnosis for smoking cessation: a randomized trial". Nicotine & Tobacco Research. 10 (5): 811–8. doi:10.1080/14622200802023833. PMID 18569754.
- Mayo Clinic. "St. John's wort (Hypericum perforatum) Evidence - Mayo Clinic". Mayo Clinic.
- Sood A, Ebbert JO, Prasad K, Croghan IT, Bauer B, Schroeder DR (July 2010). "A randomized clinical trial of St. John's wort for smoking cessation". Journal of Alternative and Complementary Medicine. 16 (7): 761–7. doi:10.1089/acm.2009.0445. PMC 3110810. PMID 20590478.
- U.S. Food and Drug Administration. "FDA Poisonous Plant Database". www.accessdata.fda.gov.
- SCENIHR. Health Effects of Smokeless Tobacco Products (PDF) (Report). p. 103.
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). "Health effects of smokeless tobacco products" (PDF).
- Popova L, Ling PM (May 2013). "Alternative tobacco product use and smoking cessation: a national study". American Journal of Public Health. 103 (5): 923–30. doi:10.2105/ajph.2012.301070. PMC 3661190. PMID 23488521.
- Hajek P, Stead LF (2004). "Aversive smoking for smoking cessation". The Cochrane Database of Systematic Reviews (3): CD000546. doi:10.1002/14651858.CD000546.pub2. PMC 7045729. PMID 15266433.
- Hartmann-Boyce J, Cahill K, Hatsukami D, Cornuz J (August 2012). "Nicotine vaccines for smoking cessation". The Cochrane Database of Systematic Reviews (8): CD007072. doi:10.1002/14651858.CD007072.pub2. PMC 6486305. PMID 22895958.
- Coughlin LN, Tegge AN, Sheffer CE, Bickel WK (December 2018). "A machine-learning approach to predicting smoking cessation treatment outcomes". Nicotine & Tobacco Research. 22 (3): 415–422. doi:10.1093/ntr/nty259. PMID 30508122.
- Sadasivam RS, Borglund EM, Adams R, Marlin BM, Houston TK (November 2016). "Impact of a Collective Intelligence Tailored Messaging System on Smoking Cessation: The Perspect Randomized Experiment". Journal of Medical Internet Research. 18 (11): e285. doi:10.2196/jmir.6465. PMC 5120237. PMID 27826134.
- Patrick H, Fujii CA, Glaser DB, Utley DS, Marler JD (December 2018). "A Comprehensive Digital Program for Smoking Cessation: Assessing Feasibility in a Single-Group Cohort Study". JMIR mHealth and uHealth. 6 (12): e11708. doi:10.2196/11708. PMC 6315234. PMID 30563807.
- Besson M, Forget B (2016). "Cognitive Dysfunction, Affective States, and Vulnerability to Nicotine Addiction: A Multifactorial Perspective". Frontiers in Psychiatry. 7: 160. doi:10.3389/fpsyt.2016.00160. PMC 5030478. PMID 27708591.

- Health, United States, 2018. . Hyattsville, MD: National Center for Health Statistics. 2019.
- Jones, Miranda R.; Joshu, Corinne E.; Navas-Acien, Ana; Platz, Elizabeth A. (21 December 2016). "Racial/Ethnic Differences in Duration of Smoking among Former Smokers in the National Health and Nutrition Examination Surveys (NHANES)". Nicotine & Tobacco Research. 20 (3): 303–311. doi:10.1093/ntr/ntw326. PMC 5896512. PMID 28003510.
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone-Banks J, Hartmann-Boyce J (November 2017). "Tobacco cessation interventions for young people". The Cochrane Database of Systematic Reviews. 11: CD003289. doi:10.1002/14651858.CD003289.pub6. PMC 6486118. PMID 29148565.
- Phert L. "Intensive Counseling of Students by School Nurses Does Not Have Larger Impact on Long-Term Smoking Rates Than Briefer Sessions | AHRQ Health Care Innovations Exchange". innovations.ahrq.gov. Retrieved 19 July 2016.
- Davis KC, Farrelly MC, Messeri P, Duke J (February 2009). "The impact of national smoking prevention campaigns on tobacco-related beliefs, intentions to smoke and smoking initiation: results from a longitudinal survey of youth in the United States". International Journal of Environmental Research and Public Health. 6 (2): 722–40. doi:10.3390/ijerph6020722. PMC 2672353. PMID 19440412.
- Allen JA, Duke JC, Davis KC, Kim AE, Nonnemaker JM, Farrelly MC (Nov–Dec 2015). "Using mass media campaigns to reduce youth tobacco use: a review". American Journal of Health Promotion. 30 (2): e71-82. doi:10.4278/ajhp.130510-lit-237. PMID 25372236.
- Carson KV, Brinn MP, Labiszewski NA, Esterman AJ, Chang AB, Smith BJ (July 2011). "Community interventions for preventing smoking in young people" (PDF). The Cochrane Database of Systematic Reviews (7): CD001291. doi:10.1002/14651858.CD001291.pub2. PMID 21735383.
- Mund M, Louwen F, Klingelhoefer D, Gerber A (November 2013). "Smoking and pregnancy — a review on the first major environmental risk factor of the unborn". International Journal of Environmental Research and Public Health. 10 (12): 6485–99. doi:10.3390/ijerph10126485. PMC 3881126. PMID 24351784.
- Anderson TM, Lavista Ferres JM, Ren SY, Moon RY, Goldstein RD, Ramirez JM, Mitchell EA (April 2019). "Maternal Smoking Before and During Pregnancy and the Risk of Sudden Unexpected Infant Death". Pediatrics. 143 (4): e20183325. doi:10.1542/peds.2018-3325. PMC 6564075. PMID 30858347.
- Chamberlain C, O'Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, McKenzie JE (February 2017). "Psychosocial interventions for supporting women to stop smoking in pregnancy". The Cochrane Database of Systematic Reviews. 2: CD001055. doi:10.1002/14651858.CD001055.pub5. PMC 4022453. PMID 28196405.
- Bowen M (25 February 2013). "Pregnancy and smoking". Netdoctor. Retrieved 19 July 2016.
- de Leon J, Diaz FJ (July 2005). "A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors". Schizophrenia Research. 76 (2–3): 135–57. doi:10.1016/j.schres.2005.02.010. PMID 15949648.
- Keltner NL, Grant JS (November 2006). "Smoke, smoke, smoke that cigarette". Perspectives in Psychiatric Care. 42 (4): 256–61. doi:10.1111/j.1744-6163.2006.00085.x. PMID 17107571.
- Cahill K, Lancaster T (February 2014). "Workplace interventions for smoking cessation". The Cochrane Database of Systematic Reviews (2): CD003440. doi:10.1002/14651858.CD003440.pub4. PMID 24570145.
- Leeks KD, Hopkins DP, Soler RE, Aten A, Chattopadhyay SK (February 2010). "Worksite-based incentives and competitions to reduce tobacco use. A systematic review" (PDF). American Journal of Preventive Medicine. 38 (2 Suppl): S263-74. doi:10.1016/j.amepre.2009.10.034. PMID 20117611.
- West R, Shiffman S (2007). Fast facts: smoking cessation (2nd ed.). Abingdon, England: Health Press Ltd. ISBN 978-1-903734-98-8.
- Rigotti NA, Clair C, Munafò MR, Stead LF (May 2012). "Interventions for smoking cessation in hospitalised patients". The Cochrane Database of Systematic Reviews (5): CD001837. doi:10.1002/14651858.CD001837.pub3. PMC 4498489. PMID 22592676.
- Thomsen T, Villebro N, Møller AM (March 2014). "Interventions for preoperative smoking cessation". The Cochrane Database of Systematic Reviews (3): CD002294. doi:10.1002/14651858.CD002294.pub4. PMC 7138216. PMID 24671929.
- Caponnetto P, Russo C, Bruno CM, Alamo A, Amaradio MD, Polosa R (March 2013). "Electronic cigarette: a possible substitute for cigarette dependence". Monaldi Archives for Chest Disease = Archivio Monaldi per le Malattie del Torace. 79 (1): 12–9. doi:10.4081/monaldi.2013.104. PMID 23741941.
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J (September 1990). "Smoking, smoking cessation, and major depression". JAMA. 264 (12): 1546–9. doi:10.1001/jama.1990.03450120058029. PMID 2395194.
- Moylan S, Jacka FN, Pasco JA, Berk M (May 2013). "How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways". Brain and Behavior. 3 (3): 302–26. doi:10.1002/brb3.137. PMC 3683289. PMID 23785661.
- Baggett TP, Lebrun-Harris LA, Rigotti NA (November 2013). "Homelessness, cigarette smoking and desire to quit: results from a US national study". Addiction. 108 (11): 2009–18. doi:10.1111/add.12292. PMC 3797258. PMID 23834157.
- "Other Vulnerable Populations | Smoking Cessation Leadership Center". smokingcessationleadership.ucsf.edu. Retrieved 2017-06-29.
- "National Coalition for the Homeless". www.nationalhomeless.org. Retrieved 2017-06-29.
- CDC's Office on Smoking and Health. "CDC - Fact Sheet - Adult Cigarette Smoking in the United States - Smoking & Tobacco Use". Smoking and Tobacco Use. Retrieved 2017-06-29.
- Baggett TP, Tobey ML, Rigotti NA (July 2013). "Tobacco use among homeless people--addressing the neglected addiction". The New England Journal of Medicine. 369 (3): 201–4. doi:10.1056/NEJMp1301935. PMID 23863048.
- Pool ER, Dogar O, Lindsay RP, Weatherburn P, Siddiqi K (June 2016). "Interventions for tobacco use cessation in people living with HIV and AIDS" (PDF). The Cochrane Database of Systematic Reviews (6): CD011120. doi:10.1002/14651858.CD011120.pub2. PMID 27292836.
- Campbell, Barbara K.; Le, Thao; Andrews, K. Blakely; Pramod, Sowmya; Guydish, Joseph (2016). "Smoking among Patients in Substance Use Disorders Treatment: Associations with Tobacco Advertising, Anti-tobacco Messages and Perceived Health Risk". The American Journal of Drug and Alcohol Abuse. 42 (6): 649–656. doi:10.1080/00952990.2016.1183021. ISSN 0095-2990. PMC 5093078. PMID 27314450.
- Apollonio, Dorie; Philipps, Rose; Bero, Lisa (2016). "Interventions for tobacco use cessation in people in treatment for or recovery from substance use disorders". The Cochrane Database of Systematic Reviews. 11: CD010274. doi:10.1002/14651858.CD010274.pub2. ISSN 1469-493X. PMC 6464324. PMID 27878808.
- Naqvi NH, Rudrauf D, Damasio H, Bechara A (January 2007). "Damage to the insula disrupts addiction to cigarette smoking". Science. 315 (5811): 531–4. Bibcode:2007Sci...315..531N. doi:10.1126/science.1135926. PMC 3698854. PMID 17255515.
- Lemmens V, Oenema A, Knut IK, Brug J (November 2008). "Effectiveness of smoking cessation interventions among adults: a systematic review of reviews". European Journal of Cancer Prevention. 17 (6): 535–44. doi:10.1097/cej.0b013e3282f75e48. PMID 18941375.
- King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET (June 2009). "Link between facultative melanin and tobacco use among African Americans" (PDF). Pharmacology, Biochemistry, and Behavior. 92 (4): 589–96. doi:10.1016/j.pbb.2009.02.011. PMID 19268687. Archived from the original on 2016-03-04. Retrieved 2012-09-12.CS1 maint: unfit url (link)
- Christakis NA, Fowler JH (May 2008). "The collective dynamics of smoking in a large social network". The New England Journal of Medicine. 358 (21): 2249–58. doi:10.1056/NEJMsa0706154. PMC 2822344. PMID 18499567.
- Faseru B, Richter KP, Scheuermann TS, Park EW (August 2018). "Enhancing partner support to improve smoking cessation". The Cochrane Database of Systematic Reviews. 8: CD002928. doi:10.1002/14651858.CD002928.pub4. PMC 6326744. PMID 30101972.
- Peoples CD, Sigillo AE, Green M, Miller MK (2012). "Friendship and Conformity in Group Opinions: Juror Verdict Change in Mock Juries". Sociological Spectrum. 32 (2): 178–193. doi:10.1080/02732173.2012.646163.
- Hitchman SC, Fong GT, Zanna MP, Thrasher JF, Laux FL (December 2014). "The relation between number of smoking friends, and quit intentions, attempts, and success: findings from the International Tobacco Control (ITC) Four Country Survey". Psychology of Addictive Behaviors. 28 (4): 1144–52. doi:10.1037/a0036483. PMC 4266625. PMID 24841185.
- American Cancer Society. "Guide to Quitting Smoking". www.cancer.org. Retrieved 6 July 2016.
- Glassman AH, Helzer JE, Covey LS, Cottler LB, Stetner F, Tipp JE, Johnson J (September 1990). "Smoking, smoking cessation, and major depression". JAMA. 264 (12): 1546–9. doi:10.1001/jama.1990.03450120058029. PMID 2395194.
- Condiotte MM, Lichtenstein E (October 1981). "Self-efficacy and relapse in smoking cessation programs". Journal of Consulting and Clinical Psychology. 49 (5): 648–58. doi:10.1037/0022-006X.49.5.648. PMID 7287974.
- Elfeddali I, Bolman C, Candel MJ, Wiers RW, De Vries H (February 2012). "The role of self-efficacy, recovery self-efficacy, and preparatory planning in predicting short-term smoking relapse". British Journal of Health Psychology. 17 (1): 185–201. doi:10.1111/j.2044-8287.2011.02032.x. PMID 22107073.
- Shiffman S (February 1982). "Relapse following smoking cessation: a situational analysis". Journal of Consulting and Clinical Psychology. 50 (1): 71–86. doi:10.1037/0022-006X.50.1.71. PMID 7056922.
- Livingstone-Banks, Jonathan; Norris, Emma; Hartmann-Boyce, Jamie; West, Robert; Jarvis, Martin; Chubb, Emma; Hajek, Peter (28 October 2019). "Relapse prevention interventions for smoking cessation". The Cochrane Database of Systematic Reviews. 2019 (10). doi:10.1002/14651858.CD003999.pub6. ISSN 1469-493X. PMC 6816175. PMID 31684681.
- Agboola SA, Coleman T, McNeill A, Leonardi-Bee J (July 2015). "Abstinence and relapse among smokers who use varenicline in a quit attempt-a pooled analysis of randomized controlled trials". Addiction. 110 (7): 1182–93. doi:10.1111/add.12941. PMID 25846123.
- Kaiser Foundation Health Plan of the Northwest (2008). Cultivating Health: Freedom From Tobacco Kit. Kaiser Permanente. ISBN 978-0-9744864-8-2.
- Hughes JR, Stead LF, Lancaster T (January 2007). Hughes JR (ed.). "Antidepressants for smoking cessation". The Cochrane Database of Systematic Reviews (1): CD000031. doi:10.1002/14651858.CD000031.pub3. PMID 17253443.
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P (July 2012). "Weight gain in smokers after quitting cigarettes: meta-analysis". BMJ. 345 (345): e4439. doi:10.1136/bmj.e4439. PMC 3393785. PMID 22782848.
- Vanni H, Kazeros A, Wang R, Harvey BG, Ferris B, De BP, Carolan BJ, Hübner RH, O'Connor TP, Crystal RG (May 2009). "Cigarette smoking induces overexpression of a fat-depleting gene AZGP1 in the human". Chest. 135 (5): 1197–1208. doi:10.1378/chest.08-1024. PMC 2679098. PMID 19188554.
- Jo YH, Talmage DA, Role LW (December 2002). "Nicotinic receptor-mediated effects on appetite and food intake". Journal of Neurobiology. 53 (4): 618–32. doi:10.1002/neu.10147. PMC 2367209. PMID 12436425.
- Klag MJ (1999). Johns Hopkins family health book. New York: HarperCollins. p. 86. ISBN 978-0-06-270149-7.
- Farley AC, Hajek P, Lycett D, Aveyard P (January 2012). "Interventions for preventing weight gain after smoking cessation". The Cochrane Database of Systematic Reviews. 1: CD006219. doi:10.1002/14651858.CD006219.pub3. PMID 22258966.
- Covey LS, Glassman AH, Stetner F (February 1997). "Major depression following smoking cessation". The American Journal of Psychiatry. 154 (2): 263–5. doi:10.1176/ajp.154.2.263. PMID 9016279.
- Shahab L, Andrew S, West R (January 2014). "Changes in prevalence of depression and anxiety following smoking cessation: results from an international cohort study (ATTEMPT)" (PDF). Psychological Medicine. 44 (1): 127–41. doi:10.1017/s0033291713000391. PMID 23507203.
- Borrelli B, Bock B, King T, Pinto B, Marcus BH (1996). "The impact of depression on smoking cessation in women". American Journal of Preventive Medicine. 12 (5): 378–87. doi:10.1016/S0749-3797(18)30295-2. PMID 8909649.
- McDermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P (January 2013). "Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study". The British Journal of Psychiatry. 202 (1): 62–7. doi:10.1192/bjp.bp.112.114389. PMID 23284151.
- Doll R, Peto R, Boreham J, Sutherland I (June 2004). "Mortality in relation to smoking: 50 years' observations on male British doctors". BMJ. 328 (7455): 1519. doi:10.1136/bmj.38142.554479.AE. PMC 437139. PMID 15213107.
- American Cancer Society. "Benefits of Quitting Smoking Over Time". www.cancer.org. Retrieved 22 July 2016.
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R (August 2000). "Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies". BMJ. 321 (7257): 323–9. doi:10.1136/bmj.321.7257.323. PMC 27446. PMID 10926586.
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE (February 2005). "The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial". Annals of Internal Medicine. 142 (4): 233–9. doi:10.7326/0003-4819-142-4-200502150-00005. PMID 15710956.
- Taghizadeh N, Vonk JM, Boezen HM (7 April 2016). "Lifetime Smoking History and Cause-Specific Mortality in a Cohort Study with 43 Years of Follow-Up". PLOS ONE. 11 (4): e0153310. Bibcode:2016PLoSO..1153310T. doi:10.1371/journal.pone.0153310. PMC 4824471. PMID 27055053.
- Mills E, Eyawo O, Lockhart I, Kelly S, Wu P, Ebbert JO (February 2011). "Smoking cessation reduces postoperative complications: a systematic review and meta-analysis". The American Journal of Medicine. 124 (2): 144–154.e8. doi:10.1016/j.amjmed.2010.09.013. PMID 21295194.
- Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T (December 1997). "Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research". JAMA. 278 (21): 1759–66. doi:10.1001/jama.278.21.1759. PMID 9388153.
- Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Mölken MP (August 2010). "Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD". Thorax. 65 (8): 711–8. doi:10.1136/thx.2009.131631. PMID 20685746.
- Bauld L, Boyd KA, Briggs AH, Chesterman J, Ferguson J, Judge K, Hiscock R (February 2011). "One-year outcomes and a cost-effectiveness analysis for smokers accessing group-based and pharmacy-led cessation services". Nicotine & Tobacco Research. 13 (2): 135–45. doi:10.1093/ntr/ntq222. PMID 21196451.
- Schiaffino A, Fernández E, Kunst A, Borrell C, García M, Borràs JM, Mackenbach JP (2007). "Time trends and educational differences in the incidence of quitting smoking in Spain (1965-2000)". Preventive Medicine. 45 (2–3): 226–32. doi:10.1016/j.ypmed.2007.05.009. PMID 17604832.
- Fowkes FJ, Stewart MC, Fowkes FG, Amos A, Price JF (November 2008). "Scottish smoke-free legislation and trends in smoking cessation". Addiction. 103 (11): 1888–95. doi:10.1111/j.1360-0443.2008.02350.x. PMID 19032538.
- Federico B, Costa G, Ricciardi W, Kunst AE (October 2009). "Educational inequalities in smoking cessation trends in Italy, 1982-2002". Tobacco Control. 18 (5): 393–8. doi:10.1136/tc.2008.029280. PMID 19617220.
- Centers for Disease Control Prevention (CDC) (November 2009). "Cigarette smoking among adults and trends in smoking cessation - United States, 2008". MMWR. Morbidity and Mortality Weekly Report. 58 (44): 1227–32. PMID 19910909.
- Qian J, Cai M, Gao J, Tang S, Xu L, Critchley JA (October 2010). "Trends in smoking and quitting in China from 1993 to 2003: National Health Service Survey data". Bulletin of the World Health Organization. 88 (10): 769–76. doi:10.2471/BLT.09.064709. PMC 2947036. PMID 20931062.
- Martin, Anya (May 13, 2010). "What it takes to quit smoking". MarketWatch. Dow Jones. p. 2. Retrieved May 14, 2010.
- Health, CDC's Office on Smoking and. "CDC - Fact Sheet - Adult Cigarette Smoking in the United States - Smoking & Tobacco Use". Smoking and Tobacco Use. Retrieved 21 July 2016.
Further reading
| Wikimedia Commons has media related to Smoking cessation. |
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML (2005). "Pharmacotherapy for nicotine dependence". Ca. 55 (5): 281–99, quiz 322–3, 325. doi:10.3322/canjclin.55.5.281. PMID 16166074.
- Hughes JR, Keely J, Naud S (January 2004). "Shape of the relapse curve and long-term abstinence among untreated smokers". Addiction. 99 (1): 29–38. doi:10.1111/j.1360-0443.2004.00540.x. PMID 14678060.
- Hutter H, Moshammer H, Neuberger M (January 2006). "Smoking cessation at the workplace: 1 year success of short seminars". International Archives of Occupational and Environmental Health. 79 (1): 42–8. doi:10.1007/s00420-005-0034-y. PMID 16133522.
- Marks DF (2005). Overcoming your smoking habit: a self-help guide using cognitive behavioral techniques. London: Robinson. ISBN 978-1-84529-067-2.
- Peters MJ, Morgan LC (May 2002). "The pharmacotherapy of smoking cessation". The Medical Journal of Australia. 176 (10): 486–90. doi:10.5694/j.1326-5377.2002.tb04521.x. PMID 12065013.
- West R (2006). "Tobacco control: present and future". British Medical Bulletin. 77–78 (1): 123–36. doi:10.1093/bmb/ldl012. PMID 17106058.
- McFarland JW, Folkenberg EJ (1964). How to stop smoking in five days (PDF). Englewood Cliffs, NJ: Prentice-Hall. Archived from the original on 2015-02-13.CS1 maint: unfit url (link)