Monocotyledon
Monocotyledons (/ˌmɒnəˌkɒtəlˈiːdən/),[lower-alpha 4][13][14] commonly referred to as monocots, (Lilianae sensu Chase & Reveal) are flowering plants (angiosperms), the seeds of which typically contain only one embryonic leaf, or cotyledon. They constitute one of the major groups into which the flowering plants have traditionally been divided, the rest of the flowering plants having two cotyledons and therefore classified as dicotyledons, or dicots. However, molecular phylogenetic research has shown that while the monocots form a monophyletic group or clade (comprising all the descendants of a common ancestor), the dicotyledons do not. Monocotyledons have almost always been recognized as a group, but with various taxonomic ranks and under several different names. The APG III system of 2009 recognises a clade called "monocots" but does not assign it to a taxonomic rank.
| Monocotyledons | |
|---|---|
| Wheat – an economically important monocotyledon | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Monocots |
| Type genus | |
| Lilium L.[1] | |
| Orders | |
| Synonyms | |
The monocotyledons include about 60,000 species. The largest family in this group (and in the flowering plants as a whole) by number of species are the orchids (family Orchidaceae), with more than 20,000 species. About half as many species belong to the true grasses (Poaceae), which are economically the most important family of monocotyledons. In agriculture the majority of the biomass produced comes from monocotyledons. These include not only major grains (rice, wheat, maize, etc.), but also forage grasses, sugar cane, and the bamboos. Other economically important monocotyledon crops include various palms (Arecaceae), bananas and plantains (Musaceae), gingers and their relatives, turmeric and cardamom (Zingiberaceae), asparagus (Asparagaceae), pineapple (Bromeliaceae), water chestnut (Cyperaceae), and leeks, onion and garlic (Amaryllidaceae). Many houseplants are monocotyledon epiphytes. Additionally most of the horticultural bulbs, plants cultivated for their blooms, such as lilies, daffodils, irises, amaryllis, cannas, bluebells and tulips, are monocotyledons.
Description

General
The monocots or monocotyledons have, as the name implies, a single (mono-) cotyledon, or embryonic leaf, in their seeds. Historically, this feature was used to contrast the monocots with the dicotyledons or dicots which typically have two cotyledons; however modern research has shown that the dicots are not a natural group, and the term can only be used to indicate all angiosperms that are not monocots and is used in that respect here. From a diagnostic point of view the number of cotyledons is neither a particularly useful characteristic (as they are only present for a very short period in a plant's life), nor is it completely reliable. The single cotyledon is only one of a number of modifications of the body plan of the ancestral monocotyledons, whose adaptive advantages are poorly understood, but may have been related to adaption to aquatic habitats, prior to radiation to terrestrial habitats. Nevertheless, monocots are sufficiently distinctive that there has rarely been disagreement as to membership of this group, despite considerable diversity in terms of external morphology.[15] However, morphological features that reliably characterise major clades are rare.[16]
Thus monocots are distinguishable from other angiosperms both in terms of their uniformity and diversity. On the one hand the organisation of the shoots, leaf structure and floral configuration are more uniform than in the remaining angiosperms, yet within these constraints a wealth of diversity exists, indicating a high degree of evolutionary success.[17] Monocot diversity includes perennial geophytes such as ornamental flowers including (orchids (Asparagales), tulips and lilies) (Liliales), rosette and succulent epiphytes (Asparagales), mycoheterotrophs (Liliales, Dioscoreales, Pandanales), all in the lilioid monocots, major cereal grains (maize, rice, barley, rye, oats, millet, sorghum and wheat) in the grass family and forage grasses (Poales) as well as woody tree-like palm trees (Arecales), bamboo, reeds and bromeliads (Poales), bananas and ginger (Zingiberales) in the commelinid monocots, as well as both emergent (Poales, Acorales) and aroids, as well as floating or submerged aquatic plants such as seagrass (Alismatales).[18][19][20][21]
Vegetative
- Organisation, growth and life forms
The most important distinction is their growth pattern, lacking a lateral meristem (cambium) that allows for continual growth in diameter with height (secondary growth), and therefore this characteristic is a basic limitation in shoot construction. Although largely herbaceous, some arboraceous monocots reach great height, length and mass. The latter include agaves, palms, pandans, and bamboos.[22][23] This creates challenges in water transport that monocots deal with in various ways. Some, such as species of Yucca, develop anomalous secondary growth, while palm trees utilise an anomalous primary growth form described as establishment growth (see Vascular system). The axis undergoes primary thickening, that progresses from internode to internode, resulting in a typical inverted conical shape of the basal primary axis (see Tillich, Figure 1). The limited conductivity also contributes to limited branching of the stems. Despite these limitations a wide variety of adaptive growth forms has resulted (Tillich, Figure 2) from epiphytic orchids (Asparagales) and bromeliads (Poales) to submarine Alismatales (including the reduced Lemnoideae) and mycotrophic Burmanniaceae (Dioscreales) and Triuridaceae (Pandanales). Other forms of adaptation include the climbing vines of Araceae (Alismatales) which use negative phototropism (skototropism) to locate host trees (i.e. the darkest area),[24] while some palms such as Calamus manan (Arecales) produce the longest shoots in the plant kingdom, up to 185 m long.[25] Other monocots, particularly Poales, have adopted a therophyte life form.[26][27][28][29][30]
Leaves
The cotyledon, the primordial Angiosperm leaf consists of a proximal leaf base or hypophyll and a distal hyperphyll. In monocots the hypophyll tends to be the dominant part in contrast to other angiosperms. From these, considerable diversity arises. Mature monocot leaves are generally narrow and linear, forming a sheathing around the stem at its base, although there are many exceptions. Leaf venation is of the striate type, mainly arcuate-striate or longitudinally striate (parallel), less often palmate-striate or pinnate-striate with the leaf veins emerging at the leaf base and then running together at the apices. There is usually only one leaf per node because the leaf base encompasses more than half the circumference.[31] The evolution of this monocot characteristic has been attributed to developmental differences in early zonal differentiation rather than meristem activity (leaf base theory).[15][16][32]
Roots and underground organs
The lack of cambium in the primary root limits its ability to grow sufficiently to maintain the plant. This necessitates early development of roots derived from the shoot (adventitious roots). In addition to roots, monocots develop runners and rhizomes, which are creeping shoots. Runners serve vegetative propagation, have elongated internodes, run on or just below the surface of the soil and in most case bear scale leaves. Rhizomes frequently have an additional storage function and rhizome producing plants are considered geophytes (Tillich, Figure 11). Other geophytes develop bulbs, a short axial body bearing leaves whose bases store food. Additional outer non-storage leaves may form a protective function (Tillich, Figure 12). Other storage organs may be tubers or corms, swollen axes. Tubers may form at the end of underground runners and persist. Corms are short lived vertical shoots with terminal inflorescences and shrivel once flowering has occurred. However, intermediate forms may occur such as in Crocosmia (Asparagales). Some monocots may also produce shoots that grow directly down into the soil, these are geophilous shoots (Tillich, Figure 11) that help overcome the limited trunk stability of large woody monocots.[33][32][34][15]
Reproductive
- Flowers
In nearly all cases the perigone consists of two alternating trimerous whorls of tepals, being homochlamydeous, without differentiation between calyx and corolla. In zoophilous (pollinated by animals) taxa, both whorls are corolline (petal-like). Anthesis (the period of flower opening) is usually fugacious (short lived). Some of the more persistent perigones demonstrate thermonastic opening and closing (responsive to changes in temperature). About two thirds of monocots are zoophilous, predominantly by insects. These plants need to advertise to pollinators and do so by way of phaneranthous (showy) flowers. Such optical signalling is usually a function of the tepal whorls but may also be provided by semaphylls (other structures such as filaments, staminodes or stylodia which have become modified to attract pollinators). However, some monocot plants may have aphananthous (inconspicuous) flowers and still be pollinated by animals. In these the plants rely either on chemical attraction or other structures such as coloured bracts fulfill the role of optical attraction. In some phaneranthous plants such structures may reinforce floral structures. The production of fragrances for olfactory signalling are common in monocots. The perigone also functions as a landing platform for pollinating insects. [17]
- Fruit and seed
The embryo consists of a single cotyledon, usually with two vascular bundles.[32]
Comparison with "dicots"


The traditionally listed differences between monocots and "dicots" are as follows. This is a broad sketch only, not invariably applicable, as there are a number of exceptions. The differences indicated are more true for monocots versus eudicots.[34][35][36]
| Feature | In monocots | In "dicots" |
|---|---|---|
| Growth form | Mostly herbaceous, occasionally arboraceous | Herbaceous or arboraceous |
| Leaves[16] | Leaf shape oblong or linear, often sheathed at base, petiole seldom developed, stipules absent. Major leaf veins usually parallel | Broad, seldom sheathed, petiole common often with stipules. Veins usually reticulate (pinnate or palmate) |
| Roots | Primary root of short duration, replaced by adventitial roots forming fibrous or fleshy root systems | Develops from the radicle. Primary root often persists forming strong taproot and secondary roots |
| Plant stem: Vascular bundles | Numerous scattered bundles in ground parenchyma, cambium rarely present, no differentiation between cortical and stelar regions | Ring of primary bundles with cambium, differentiated into cortex and stele (eustelic) |
| Flowers | Parts in threes (trimerous) or multiples of three (e.g. 3, 6 or 9 petals) | Fours (tetramerous) or fives (pentamerous) |
| Pollen: Number of apertures (furrows or pores) | Monocolpate (single aperture or colpus) | Tricolpate (three) |
| Embryo: Number of cotyledons (leaves in the seed) | One, endosperm frequently present in seed | Two, endosperm present or absent |
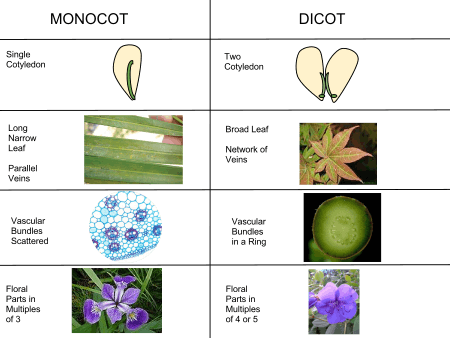
A number of these differences are not unique to the monocots, and, while still useful, no one single feature will infallibly identify a plant as a monocot.[35] For example, trimerous flowers and monosulcate pollen are also found in magnoliids,[34] and exclusively adventitious roots are found in some of the Piperaceae.[34] Similarly, at least one of these traits, parallel leaf veins, is far from universal among the monocots. Broad leaves and reticulate leaf veins, features typical of dicots, are found in a wide variety of monocot families: for example, Trillium, Smilax (greenbriar), Pogonia (an orchid), and the Dioscoreales (yams).[34] Potamogeton and Paris quadrifolia (herb-paris) are examples of monocots with tetramerous flowers. Other plants exhibit a mixture of characteristics. Nymphaeaceae (water lilies) have reticulate veins, a single cotyledon, adventitious roots, and a monocot-like vascular bundle. These examples reflect their shared ancestry.[35] Nevertheless, this list of traits is generally valid, especially when contrasting monocots with eudicots, rather than non-monocot flowering plants in general.[34]
Apomorphies
Monocot apomorphies (characteristics derived during radiation rather than inherited from an ancestral form) include herbaceous habit, leaves with parallel venation and sheathed base, an embryo with a single cotyledon, an atactostele, numerous adventitious roots, sympodial growth, and trimerous (3 parts per whorl) flowers that are pentacyclic (5 whorled) with 3 sepals, 3 petals, 2 whorls of 3 stamens each, and 3 carpels. In contrast, monosulcate pollen is considered an ancestral trait, probably plesiomorphic.[36]
Synapomorphies
The distinctive features of the monocots have contributed to the relative taxonomic stability of the group. Douglas E. Soltis and others[37][38][39][40] identify thirteen synapomorphies (shared characteristics that unite monophyletic groups of taxa);
- Calcium oxalate raphides
- Absence of vessels in leaves
- Monocotyledonous anther wall formation*
- Successive microsporogenesis
- Syncarpous gynoecium
- Parietal placentation
- Monocotyledonous seedling
- Persistent radicle
- Haustorial cotyledon tip[41]
- Open cotyledon sheath
- Steroidal saponins*
- Fly pollination*
- Diffuse vascular bundles and absence of secondary growth[lower-alpha 6]
Vascular system

Monocots have a distinctive arrangement of vascular tissue known as an atactostele in which the vascular tissue is scattered rather than arranged in concentric rings. Collenchyma is absent in monocot stems, roots and leaves. Many monocots are herbaceous and do not have the ability to increase the width of a stem (secondary growth) via the same kind of vascular cambium found in non-monocot woody plants.[34] However, some monocots do have secondary growth; because this does not arise from a single vascular cambium producing xylem inwards and phloem outwards, it is termed "anomalous secondary growth".[42] Examples of large monocots which either exhibit secondary growth, or can reach large sizes without it, are palms (Arecaceae), screwpines (Pandanaceae), bananas (Musaceae), Yucca, Aloe, Dracaena, and Cordyline.[34]
Taxonomy
The monocots form one of five major lineages of mesangiosperms (core angiosperms), which in themselves form 99.95% of all angiosperms. The monocots and the eudicots, are the largest and most diversified angiosperm radiations accounting for 22.8% and 74.2% of all angiosperm species respectively.[43]
Of these, the grass family (Poaceae) is the most economically important, which together with the orchids Orchidaceae account for half of the species diversity, accounting for 34% and 17% of all monocots respectively and are among the largest families of angiosperms. They are also among the dominant members of many plant communities.[43]
Early history
Pre-Linnean
The monocots are one of the major divisions of the flowering plants or angiosperms. They have been recognized as a natural group since the sixteenth century when Lobelius (1571), searching for a characteristic to group plants by, decided on leaf form and their venation. He observed that the majority had broad leaves with net-like venation, but a smaller group were grass-like plants with long straight parallel veins.[44] In doing so he distinguished between the dicotyledons, and the latter (grass-like) monocotyledon group, although he had no formal names for the two groups.[45][46][47]
Formal description dates from John Ray's studies of seed structure in the 17th century. Ray, who is often considered the first botanical systematist,[48] observed the dichotomy of cotyledon structure in his examination of seeds. He reported his findings in a paper read to the Royal Society on 17 December 1674, entitled "A Discourse on the Seeds of Plants".[34]
The greatest number of plants that come of seed spring at first out of the earth with two leaves which being for the most part of a different figure from the succeeding leaves are by our gardeners not improperly called the seed leaves...
In the first kind the seed leaves are nothing but the two lobes of the seed having their plain sides clapt together like the two halfs of a walnut and therefore are of the just figure of the seed slit in sunder flat wise...
Of seeds that spring out of the earth with leaves like the succeeding and no seed leaves I have observed two sorts. 1. Such as are congenerous to the first kind precedent that is whose pulp is divided into two lobes and a radicle...
2. Such which neither spring out of the ground with seed leaves nor have their pulp divided into lobes
John Ray (1674), pp. 164, 166[49]
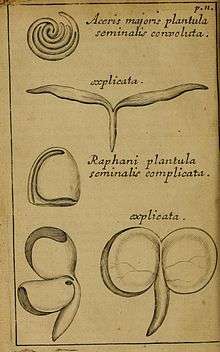
Since this paper appeared a year before the publication of Malpighi's Anatome Plantarum (1675–1679), Ray has the priority. At the time, Ray did not fully realise the importance of his discovery[50] but progressively developed this over successive publications. And since these were in Latin, "seed leaves" became folia seminalia[51] and then cotyledon, following Malpighi.[52][53] Malpighi and Ray were familiar with each other's work,[50] and Malpighi in describing the same structures had introduced the term cotyledon,[54] which Ray adopted in his subsequent writing.
In this experiment, Malpighi also showed that the cotyledons were critical to the development of the plant, proof that Ray required for his theory.[55] In his Methodus plantarum nova[56] Ray also developed and justified the "natural" or pre-evolutionary approach to classification, based on characteristics selected a posteriori in order to group together taxa that have the greatest number of shared characteristics. This approach, also referred to as polythetic would last till evolutionary theory enabled Eichler to develop the phyletic system that superseded it in the late nineteenth century, based on an understanding of the acquisition of characteristics.[57][58][59] He also made the crucial observation Ex hac seminum divisione sumum potest generalis plantarum distinctio, eaque meo judicio omnium prima et longe optima, in eas sci. quae plantula seminali sunt bifolia aut διλόβω, et quae plantula sem. adulta analoga. (From this division of the seeds derives a general distinction amongst plants, that in my judgement is first and by far the best, into those seed plants which are bifoliate, or bilobed, and those that are analogous to the adult), that is between monocots and dicots.[60][55] He illustrated this by quoting from Malpighi and including reproductions of Malpighi's drawings of cotyledons (see figure).[61] Initially Ray did not develop a classification of flowering plants (florifera) based on a division by the number of cotyledons, but developed his ideas over successive publications,[62] coining the terms Monocotyledones and Dicotyledones in 1703,[63] in the revised version of his Methodus (Methodus plantarum emendata), as a primary method for dividing them, Herbae floriferae, dividi possunt, ut diximus, in Monocotyledones & Dicotyledones (Flowering plants, can be divided, as we have said, into Monocotyledons & Dicotyledons).[64]
Post Linnean
Although Linnaeus (1707–1778) did not utilise Ray's discovery, basing his own classification solely on floral reproductive morphology, the term was used shortly after his classification appeared (1753) by Scopoli and who is credited for its introduction.[lower-alpha 7] Every taxonomist since then, starting with De Jussieu and De Candolle, has used Ray's distinction as a major classification characteristic.[lower-alpha 8][33] In De Jussieu's system (1789), he followed Ray, arranging his Monocotyledones into three classes based on stamen position and placing them between Acotyledones and Dicotyledones.[68] De Candolle's system (1813) which was to predominate thinking through much of the 19th century used a similar general arrangement, with two subgroups of his Monocotylédonés (Monocotyledoneae).[3] Lindley (1830) followed De Candolle in using the terms Monocotyledon and Endogenae[lower-alpha 9] interchangeably. They considered the monocotyledons to be a group of vascular plants (Vasculares) whose vascular bundles were thought to arise from within (Endogènes or endogenous).[69]
Monocotyledons remained in a similar position as a major division of the flowering plants throughout the nineteenth century, with minor variations. George Bentham and Hooker (1862–1883) used Monocotyledones, as would Wettstein,[70] while August Eichler used Mononocotyleae[10] and Engler, following de Candolle, Monocotyledoneae.[71] In the twentieth century, some authors used alternative names such as Bessey's (1915) Alternifoliae[2] and Cronquist's (1966) Liliatae.[1] Later (1981) Cronquist changed Liliatae to Liliopsida,[72] usages also adopted by Takhtajan simultaneously.[32] Thorne (1992)[8] and Dahlgren (1985)[73] also used Liliidae as a synonym.
Taxonomists had considerable latitude in naming this group, as the Monocotyledons were a group above the rank of family. Article 16 of the ICBN allows either a descriptive name or a name formed from the name of an included family.
In summary they have been variously named, as follows:
- class Monocotyledoneae in the de Candolle system and the Engler system
- class Monocotyledones in the Bentham & Hooker system and the Wettstein system
- class Monocotyleae in the Eichler system
- class Liliatae then Liliopsida in the Takhtajan system and the Cronquist system
- subclass Liliidae in the Dahlgren system and the Thorne system
Modern era
Over the 1980s, a more general review of the classification of angiosperms was undertaken. The 1990s saw considerable progress in plant phylogenetics and cladistic theory, initially based on rbcL gene sequencing and cladistic analysis, enabling a phylogenetic tree to be constructed for the flowering plants.[74] The establishment of major new clades necessitated a departure from the older but widely used classifications such as Cronquist and Thorne, based largely on morphology rather than genetic data. These developments complicated discussions on plant evolution and necessitated a major taxonomic restructuring.[75][76]
This DNA based molecular phylogenetic research confirmed on the one hand that the monocots remained as a well defined monophyletic group or clade, in contrast to the other historical divisions of the flowering plants, which had to be substantially reorganized.[34] No longer could the angiosperms be simply divided into monocotyledons and dicotyledons; it was apparent that the monocotyledons were but one of a relatively large number of defined groups within the angiosperms.[77] Correlation with morphological criteria showed that the defining feature was not cotyledon number but the separation of angiosperms into two major pollen types, uniaperturate (monosulcate and monosulcate-derived) and triaperturate (tricolpate and tricolpate-derived), with the monocots situated within the uniaperturate groups.[74] The formal taxonomic ranking of Monoctyledons thus became replaced with monocots as an informal clade.[78][34] This is the name that has been most commonly used since the publication of the Angiosperm Phylogeny Group (APG) system in 1998 and regularly updated since.[75][79][76][80][81][82]
Within the angiosperms, there are two major grades, a small early branching basal grade, the basal angiosperms (ANA grade) with three lineages and a larger late branching grade, the core angiosperms (mesangiosperms) with five lineages, as shown in the cladogram.
Cladogram I: Phylogenetic position of the monocots within the angiosperms in APG IV (2016)[82]
|
Subdivision
While the monocotyledons have remained extremely stable in their outer borders as a well-defined and coherent monophylectic group, the deeper internal relationships have undergone considerable flux, with many competing classification systems over time.[33]
Historically, Bentham (1877), considered the monocots to consist of four alliances, Epigynae, Coronariae, Nudiflorae and Glumales, based on floral characteristics. He describes the attempts to subdivide the group since the days of Lindley as largely unsuccessful.[83] Like most subsequent classification systems it failed to distinguish between two major orders, Liliales and Asparagales, now recognised as quite separate.[84] A major advance in this respect was the work of Rolf Dahlgren (1980),[85] which would form the basis of the Angiosperm Phylogeny Group's (APG) subsequent modern classification of monocot families. Dahlgren who used the alternate name Lilliidae considered the monocots as a subclass of angiosperms characterised by a single cotyledon and the presence of triangular protein bodies in the sieve tube plastids. He divided the monocots into seven superorders, Alismatiflorae, Ariflorae, Triuridiflorae, Liliiflorae, Zingiberiflorae, Commeliniflorae and Areciflorae. With respect to the specific issue regarding Liliales and Asparagales, Dahlgren followed Huber (1969)[86] in adopting a splitter approach, in contrast to the longstanding tendency to view Liliaceae as a very broad sensu lato family. Following Dahlgren's untimely death in 1987, his work was continued by his widow, Gertrud Dahlgren, who published a revised version of the classification in 1989. In this scheme the suffix -florae was replaced with -anae (e.g. Alismatanae) and the number of superorders expanded to ten with the addition of Bromelianae, Cyclanthanae and Pandananae.[87]
Molecular studies have both confirmed the monophyly of the monocots and helped elucidate relationships within this group. The APG system does not assign the monocots to a taxonomic rank, instead recognizing a monocots clade.[88][89][90][91] However, there has remained some uncertainty regarding the exact relationships between the major lineages, with a number of competing models (including APG).[21]
The APG system establishes eleven orders of monocots.[92][82] These form three grades, the alismatid monocots, lilioid monocots and the commelinid monocots by order of branching, from early to late. In the following cladogram numbers indicate crown group (most recent common ancestor of the sampled species of the clade of interest) divergence times in mya (million years ago).[93]
Cladogram 2: The phylogenetic composition of the monocots[82]
|
Of some 70,000 species,[94] by far the largest number (65%) are found in two families, the orchids and grasses. The orchids (Orchidaceae, Asparagales) contain about 25,000 species and the grasses (Poaceae, Poales) about 11,000. Other well known groups within the Poales order include the Cyperaceae (sedges) and Juncaceae (rushes), and the monocots also include familiar families such as the palms (Arecaceae, Arecales) and lilies (Liliaceae, Liliales).[84][95]
Evolution
In prephyletic classification systems monocots were generally positioned between plants other than angiosperms and dicots, implying that monocots were more primitive. With the introduction of phyletic thinking in taxonomy (from the system of Eichler 1875–1878 onwards) the predominant theory of monocot origins was the ranalean (ranalian) theory, particularly in the work of Bessey (1915),[2] which traced the origin of all flowering plants to a Ranalean type, and reversed the sequence making dicots the more primitive group.[33]
The monocots form a monophyletic group arising early in the history of the flowering plants, but the fossil record is meagre.[96] The earliest fossils presumed to be monocot remains date from the early Cretaceous period. For a very long time, fossils of palm trees were believed to be the oldest monocots,[97] first appearing 90 million years ago (mya), but this estimate may not be entirely true.[98] At least some putative monocot fossils have been found in strata as old as the eudicots.[99] The oldest fossils that are unequivocally monocots are pollen from the Late Barremian–Aptian – Early Cretaceous period, about 120-110 million years ago, and are assignable to clade-Pothoideae-Monstereae Araceae; being Araceae, sister to other Alismatales.[100][101][102] They have also found flower fossils of Triuridaceae (Pandanales) in Upper Cretaceous rocks in New Jersey,[100] becoming the oldest known sighting of saprophytic/mycotrophic habits in angiosperm plants and among the oldest known fossils of monocotyledons.
Topology of the angiosperm phylogenetic tree could infer that the monocots would be among the oldest lineages of angiosperms, which would support the theory that they are just as old as the eudicots. The pollen of the eudicots dates back 125 million years, so the lineage of monocots should be that old too.[43]
Molecular clock estimates
Kåre Bremer, using rbcL sequences and the mean path length method for estimating divergence times, estimated the age of the monocot crown group (i.e. the time at which the ancestor of today's Acorus diverged from the rest of the group) as 134 million years.[103][104] Similarly, Wikström et al.,[105] using Sanderson's non-parametric rate smoothing approach,[106] obtained ages of 127–141 million years for the crown group of monocots.[107] All these estimates have large error ranges (usually 15-20%), and Wikström et al. used only a single calibration point,[105] namely the split between Fagales and Cucurbitales, which was set to 84 Ma, in the late Santonian period. Early molecular clock studies using strict clock models had estimated the monocot crown age to 200 ± 20 million years ago[108] or 160 ± 16 million years,[109] while studies using relaxed clocks have obtained 135-131 million years[110] or 133.8 to 124 million years.[111] Bremer's estimate of 134 million years[103] has been used as a secondary calibration point in other analyses.[112] Some estimates place the emergence of the monocots as far back as 150 mya in the Jurassic period.[21]
Core group
The age of the core group of so-called 'nuclear monocots' or 'core monocots', which correspond to all orders except Acorales and Alismatales,[113] is about 131 million years to present, and crown group age is about 126 million years to the present. The subsequent branching in this part of the tree (i.e. Petrosaviaceae, Dioscoreales + Pandanales and Liliales clades appeared), including the crown Petrosaviaceae group may be in the period around 125–120 million years BC (about 111 million years so far[103]), and stem groups of all other orders, including Commelinidae would have diverged about or shortly after 115 million years.[112] These and many clades within these orders may have originated in southern Gondwana, i.e. Antarctica, Australasia, and southern South America.[114]
Aquatic monocots
The aquatic monocots of Alismatales have commonly been regarded as "primitive".[115][116][117][72][118][119][120][121][122] They have also been considered to have the most primitive foliage, which were cross-linked as Dioscoreales[73] and Melanthiales.[8][123] Keep in mind that the "most primitive" monocot is not necessarily "the sister of everyone else".[43] This is because the ancestral or primitive characters are inferred by means of the reconstruction of character states, with the help of the phylogenetic tree. So primitive characters of monocots may be present in some derived groups. On the other hand, the basal taxa may exhibit many morphological autapomorphies. So although Acoraceae is the sister group to the remaining monocotyledons, the result does not imply that Acoraceae is "the most primitive monocot" in terms of its character states. In fact, Acoraceae is highly derived in many morphological characters, and that is precisely why Acoraceae and Alismatales occupied relatively derived positions in the trees produced by Chase et al.[88] and others.[39][124]
Some authors support the idea of an aquatic phase as the origin of monocots.[125] The phylogenetic position of Alismatales (many water), which occupy a relationship with the rest except the Acoraceae, do not rule out the idea, because it could be 'the most primitive monocots' but not 'the most basal'. The Atactostele stem, the long and linear leaves, the absence of secondary growth (see the biomechanics of living in the water), roots in groups instead of a single root branching (related to the nature of the substrate), including sympodial use, are consistent with a water source. However, while monocots were sisters of the aquatic Ceratophyllales, or their origin is related to the adoption of some form of aquatic habit, it would not help much to the understanding of how it evolved to develop their distinctive anatomical features: the monocots seem so different from the rest of angiosperms and it's difficult to relate their morphology, anatomy and development and those of broad-leaved angiosperms.[126][127]
Other taxa
In the past, taxa which had petiolate leaves with reticulate venation were considered "primitive" within the monocots, because of the superficial resemblance to the leaves of dicotyledons. Recent work suggests that while these taxa are sparse in the phylogenetic tree of monocots, such as fleshy fruited taxa (excluding taxa with aril seeds dispersed by ants), the two features would be adapted to conditions that evolved together regardless.[67][128][129][130] Among the taxa involved were Smilax, Trillium (Liliales), Dioscorea (Dioscoreales), etc. A number of these plants are vines that tend to live in shaded habitats for at least part of their lives, and this fact may also relate to their shapeless stomata.[131] Reticulate venation seems to have appeared at least 26 times in monocots, and fleshy fruits have appeared 21 times (sometimes lost later); the two characteristics, though different, showed strong signs of a tendency to be good or bad in tandem, a phenomenon described as "concerted convergence" ("coordinated convergence").[129][130]
Ecology
Emergence
Some monocots, such as grasses, have hypogeal emergence, where the mesocotyl elongates and pushes the coleoptile (which encloses and protects the shoot tip) toward the soil surface.[132] Since elongation occurs above the cotyledon, it is left in place in the soil where it was planted. Many dicots have epigeal emergence, in which the hypocotyl elongates and becomes arched in the soil. As the hypocotyl continues to elongate, it pulls the cotyledons upward, above the soil surface.
Conservation
The IUCN Red List describes four species as extinct, four as extinct in the wild, 626 as possibly extinct, 423 as critically endangered, 632 endangered, 621 vulnerable, and 269 near threatened of 4,492 whose status is known.[133]
Uses
Monocots are among the most important plants economically and culturally, and account for most of the staple foods of the world, such as cereal grains and starchy root crops, and palms, orchids and lilies, building materials, and many medicines.[43] Of the monocots, the grasses are of enormous economic importance as a source of animal and human food,[84] and form the largest component of agricultural species in terms of biomass produced.[95][134]
See also
Notes
- In 1964, Takhtajan proposed that classes including Monocotyledons, be formally named with the suffix -atae, so that the principle of typification resulted in Liliatae for monocotyledons.[6] The proposal was formally described in 1966 by Cronquist, Takhtajan and Zimmermann,[1] from which is derived the descriptor "liliates".
- Tropicos gives an earlier authority, J.H. Schaffn. 1911[7]
- Cronquist[1] attributes this term to De Candolle as DC. 1818 Syst. 1: 122[12]
- An Anglo-Latin pronunciation. OED: "Monocotyledon"
- Monocots show hypogeal development in which the cotyledon remains invisible within the seed, underground. The visible part is the first true leaf produced from the meristem
- Scopoli, in his treatment of Linnaeus' scheme comments in the Hexandria polygynia on the fact that Alisma is a member of the Gens monocotyledon[65]
- See also Lindley's review of classification systems up to 1853,[66] and Dahlgren's from 1853–1982[67]
- Endogènes (ενδον within + γεναω I create)
Citations
- Cronquist, Takhtajan & Zimmermann 1966.
- Bessey 1915.
- de Candolle 1819.
- Tropicos 2015, Lilianae
- Takhtajan 1966.
- Takhtajan 1964.
- Tropicos 2015, Liliidae
- Thorne 1992a.
- Tropicos 2015, Liliopsida
- Eichler 1886.
- Tropicos 2015, Monocotylondoneae
- de Candolle 1818–1821.
- "Monocotyledon". Merriam-Webster Dictionary.
- "Monocotyledon". Dictionary.com Unabridged. Random House.
- Tillich 1998.
- Rudall & Buzgo 2002.
- Vogel 1998.
- Kubitzki & Huber 1998.
- Kubitzki 1998.
- Davis et al. 2013.
- Zeng et al 2014.
- Du et al 2016.
- Soltis & Soltis 2016.
- Strong & Ray 1975.
- Dransfield 1978.
- Tillich 1998, Figure 1
- Mauseth 2017, Anomalous forms of growth pp. 211–219
- Petit et al 2014.
- Tomlinson & Esler 1973.
- Leck et al 2008.
- Tomlinson 1970.
- Takhtajan 2009, Liliopsida pp. 589–750
- Kubitzki, Rudall & Chase 1998, A brief history of monocot classification p. 23
- Chase 2004.
- NBGI 2016, Monocots versus Dicots.
- Stevens 2015.
- Soltis et al. 2005, p. 92.
- Donoghue & Doyle 1989b.
- Loconte & Stevenson 1991.
- Doyle & Donoghue 1992.
- Lersten 2004.
- Donoghue 2005.
- Soltis et al. 2005.
- l'Obel 1571, p. 65
- Vines 1913, p. 10.
- Hoeniger & Hoeniger 1969.
- Pavord 2005, p. 339
- Pavord 2005.
- Ray 1674, pp. 164, 166.
- Raven 1950.
- Ray 1682, De foliis plantarum seminalibus dictis p. 7.
- Short & George 2013, p. 15.
- Ray 1682, De plantula seminali reliquisque femine contentis p. 13.
- Malpighi 1679, De seminum vegetatione p. 18.
- Bewley, Black & Halmer 2006, History of seed research p. 334.
- Ray 1682.
- Stuessy 2009, Natural classification p. 47.
- Datta 1988, Systems of classification p. 21.
- Stace 1989, The development of plant taxonomy p. 17.
- Raven 1950, p. 195.
- Ray 1682, De foliis plantarum seminalibus dictis p. 11.
- Ray 1696.
- Ray 1703, pp. 1–2.
- Ray 1703, p. 16.
- Scopoli 1772, Alisma pp. 266–267
- Lindley 1853.
- Dahlgren & Clifford 1982.
- Jussieu 1789.
- Lindley 1830.
- Wettstein 1924.
- Engler 1886.
- Cronquist 1981.
- Dahlgren, Clifford & Yeo 1985.
- Chase et al 1993.
- APG 1998.
- APG III 2009.
- Bremer & Wanntorp 1978.
- Chase et al. 1995b.
- APG II 2003.
- LAPGIII 2009.
- Chase & Reveal 2009.
- APG IV 2016.
- Bentham 1877.
- Fay 2013.
- Dahlgren 1980.
- Huber 1969.
- Dahlgren 1989.
- Chase et al 1995.
- Chase et al 2000.
- Davis et al 2004.
- Soltis & Soltis 2004.
- Cantino et al 2007.
- Hertwick et al. 2015.
- CoL 2015, Liliopsida
- Panis 2008.
- Ganfolfo et al 1998.
- Smith et al 2010, p. 38.
- Herendeen & Crane 1995.
- Herendeen, Crane & Drinnan 1995.
- Gandolfo, Nixon & Crepet 2002.
- Friis, Pedersen & Crane 2004.
- Friis, Pedersen & Crane 2006.
- Bremer 2000.
- Bremer 2002.
- Wikström, Savolainen & Chase 2001.
- Sanderson 1997.
- Sanderson et al 2004.
- Savard et al 1994.
- Goremykin, Hansman & Martin 1997.
- Leebens-Mack et al 2005.
- Moore et al 2007.
- Janssen & Bremer 2004.
- Hedges & Kumar 2009, p. 205.
- Bremer & Janssen 2006.
- Hallier 1905.
- Arber 1925.
- Hutchinson 1973.
- Cronquist 1988.
- Takhtajan 2009.
- Takhtajan 1991.
- Stebbins 1974.
- Thorne 1976.
- Thorne 1992b.
- Stevenson & Loconte 1995.
- Henslow 1893.
- Zimmermann & Tomlinson 1972.
- Tomlinson 1995.
- Patterson & Givnish 2002.
- Givnish et al. 2005.
- Givnish et al. 2006.
- Cameron & Dickison 1998.
- Radosevich et al 1997, p. 149.
- IUCN 2016, Red List summary: All plant classes and families
- Tang et al 2016.
Bibliography
Books
Historical
- Batsch, August Johann Georg Karl (1802). Tabula affinitatum regni vegetabilis, quam delineavit, et nunc ulterius adumbratam tradit A.J.G.C. Batsch ... (in Latin). Weimar: Landes-Industrie-Comptoir.
- Bentham, G.; Hooker, J.D. (1862–1883). Genera plantarum ad exemplaria imprimis in herbariis kewensibus servata definita (in Latin). London: L Reeve & Co.CS1 maint: ref=harv (link)
- Birch, Thomas, ed. (1757). The History of the Royal Society of London for Improving of Natural Knowledge from Its First Rise, in which the Most Considerable of Those Papers Communicated to the Society, which Have Hitherto Not Been Published, are Inserted as a Supplement to the Philosophical Transactions, Volume 3. London: Millar.CS1 maint: ref=harv (link)
- de Candolle, Augustin Pyramus (1818–1821). Regni vegetabilis systema naturale, sive Ordines, genera et species plantarum secundum methodi naturalis normas digestarum et descriptarum 2 vols. Paris: Treuttel et Würtz.CS1 maint: ref=harv (link)
- de Candolle, AP (1819) [1813]. Théorie élémentaire de la botanique, ou exposition des principes de la classification naturelle et de l'art de décrire et d'etudier les végétaux (2nd ed.).CS1 maint: ref=harv (link)
- Eichler, August W. (1886) [1876]. Syllabus der Vorlesungen über specielle und medicinisch-pharmaceutische Botanik (4th ed.). Berlin: Borntraeger.CS1 maint: ref=harv (link)
- Engler, Adolf (1886). Führer durch den Königlich botanischen Garten der Universität zu Breslau (in German). J.U. Kerns Verlag (Max Müller). Retrieved 2 May 2015.CS1 maint: ref=harv (link)
- Jussieu, Antoine Laurent de (1789). Genera Plantarum, secundum ordines naturales disposita juxta methodum in Horto Regio Parisiensi exaratam. Paris. OCLC 5161409.CS1 maint: ref=harv (link)
- Lindley, John (1830). An introduction to the natural system of botany: or, A systematic view of the organisation, natural affinities, and geographical distribution, of the whole vegetable kingdom: together with the uses of the most important species in medicine, the arts, and rural or domestic economy (1st ed.). London: Longman.CS1 maint: ref=harv (link)
- Lindley, John (1853) [1846]. The Vegetable Kingdom: or, The structure, classification, and uses of plants, illustrated upon the natural system (3rd. ed.). London: Bradbury & Evans.CS1 maint: ref=harv (link)
- l'Obel, Matthias de (1571). Stirpium adversaria nova [A new notebook of plants]. London: Thomae Purfoetii.CS1 maint: ref=harv (link)
- Malpighi, Marcello (1675). Anatome plantarum: Cui subjungitur appendix, iteratas & auctas ejusdem authoris de ovo incubato observationes continens (in Latin). London: Johannis Martyn. Retrieved 13 December 2015.
- Malpighi, Marcello (1679). Anatome plantarum: Pars altera (in Latin). London: Johannis Martyn. Retrieved 13 December 2015.CS1 maint: ref=harv (link)
- Ray, John (1682). Methodus plantarum nova: brevitatis & perspicuitatis causa synoptice in tabulis exhibita, cum notis generum tum summorum tum subalternorum characteristicis, observationibus nonnullis de seminibus plantarum & indice copioso (in Latin). London: Faithorne & Kersey.CS1 maint: ref=harv (link)
- Ray, John (1696). De Variis Plantarum Methodis Dissertatio Brevis (in Latin). London: Smith & Walford.CS1 maint: ref=harv (link)
- Ray, John (1703). Methodus plantarum emendata et aucta: In quãa notae maxime characteristicae exhibentur, quibus stirpium genera tum summa, tum infima cognoscuntur & áa se mutuo dignoscuntur, non necessariis omissis. Accedit methodus graminum, juncorum et cyperorum specialis (in Latin). London: Smith & Walford.CS1 maint: ref=harv (link)
- Sachs, Julius von (1875). Geschichte der Botanik vom 16. Jahrhundert bis 1860 (in German). Munich: Oldenbourg. Retrieved 13 December 2015.
- Sachs, Julius von (1890) [1875]. Geschichte der Botanik vom 16. Jahrhundert bis 1860 [History of botany (1530-1860)]. translated by Henry E. F. Garnsey, revised by Isaac Bayley Balfour. Oxford: Oxford University Press. doi:10.5962/bhl.title.30585. Retrieved 13 December 2015., see also History of botany (1530-1860) at Google Books
- Scopoli, Giovanni Antonio (1772). Flora Carniolica exhibens plantas Carnioliae indigenas et distributas in classes, genera, species, varietates, ordine Linnaeano. Vindobonensis (Vienna): Ioannis Pauli Krauss.CS1 maint: ref=harv (link)
Modern
- Arber, Agnes (1925). Monocotyledons: a morphological study. Cambridge: Cambridge University Press.CS1 maint: ref=harv (link)
- Bell, Adrian D. (2008) [1991]. Plant Form. An illustrated guide to flowering plant morphology. Oxford University Press. ISBN 9780881928501.
- 1st edition ISBN 9780198542193
- Bewley, J.Derek; Black, Michael; Halmer, Peter, eds. (2006). The encyclopedia of seeds: science, technology and uses. Wallingford: CABI. ISBN 978-0-85199-723-0. Retrieved 15 December 2015.CS1 maint: ref=harv (link)
- Crane, Peter R.; Blackmore, Stephen, eds. (1989). Evolution, Systematics, and Fossil History of Hamamelidae. vol. I. Oxford: Clarendon Press. Retrieved 14 December 2015.CS1 maint: ref=harv (link)
- Cronk, Quentin C.B.; Bateman, Richard M.; Hawkins, Julie A., eds. (2002). Developmental genetics and plant evolution. London: Taylor & Francis. ISBN 9781420024982.
- Cronquist, Arthur (1981). An integrated system of classification of flowering plants. New York: Columbia University Press. ISBN 978-0-231-03880-5.CS1 maint: ref=harv (link)
- Cronquist, Arthur (1988) [1968]. The evolution and classification of flowering plants (2nd ed.). Bronx, N.Y., USA: New York Botanical Garden. ISBN 9780893273323.CS1 maint: ref=harv (link)
- Dahlgren, Rolf; Clifford, H. T. (1982). The monocotyledons: A comparative study. London and New York: Academic Press. ISBN 9780122006807.CS1 maint: ref=harv (link)
- Dahlgren, R.M.; Clifford, H.T.; Yeo, P.F. (1985). The families of the monocotyledons. Berlin: Springer-Verlag. ISBN 978-3-642-64903-5. Retrieved 10 February 2014.CS1 maint: ref=harv (link)
- Datta, Subhash Chandra (1988). Systematic Botany (4 ed.). New Delhi: New Age Intl. ISBN 81-224-0013-2. Retrieved 25 January 2015.CS1 maint: ref=harv (link)
- Fernholm, Bo; Bremer, Kåre; Jörnvall, Hans, eds. (1989). The hierarchy of life: molecules and morphology in phylogenetic analysis: proceedings from Nobel symposium 70 held at Alfred Nobel's Björkborn, Karlskoga, Sweden, August 29-September 2, 1988. Amsterdam: Excerpta Medica. ISBN 9780444810731.CS1 maint: ref=harv (link)
- Hedges, S. Blair; Kumar, Sudhir, eds. (2009), The timetree of life, Oxford: Oxford University Press, ISBN 9780191560156
- Hoeniger, F. David; Hoeniger, J. F. M. (1969). The Development of Natural History in Tudor England. MIT Press. ISBN 978-0-918016-29-4.CS1 maint: ref=harv (link)
- Hutchinson, John (1973). The families of flowering plants, arranged according to a new system based on their probable phylogeny. 2 vols (3rd ed.). Oxford: Oxford University Press. ISBN 9783874291606.CS1 maint: ref=harv (link)
- Kubitzki, Klaus; Huber, Herbert, eds. (1998). The families and genera of vascular plants. Vol.3. Flowering plants. Monocotyledons: Lilianae (except Orchidaceae). Berlin, Germany: Springer-Verlag. ISBN 3-540-64060-6. Retrieved 14 January 2014.CS1 maint: ref=harv (link)
- Kubitzki, Klaus, ed. (1998). The families and genera of vascular plants. Vol. 4. Flowering Plants. Monocotyledons: Alismatanae and Commelinanae (except Gramineae). Berlin: Springer Berlin Heidelberg. ISBN 978-3-662-03531-3.CS1 maint: ref=harv (link)
- Leck, Mary Allessio; Parker, V. Thomas; Simpson, Robert L., eds. (2008). Seedling ecology and evolution. Cambridge: Cambridge University Press. ISBN 9780521873055.
- Lersten, Nels R. (2004). Flowering plant embryology with emphasis on economic species. Ames, Iowa: Blackwell Pub. ISBN 9780470752678.CS1 maint: ref=harv (link)
- Mauseth, James D. (2017) [1991]. Botany: An Introduction to Plant Biology (6th ed.). Sudbury, MA: Jones & Bartlett. ISBN 9781284077537.CS1 maint: ref=harv (link)
- Oliver, Francis W., ed. (1913). Makers of British Botany. Cambridge: Cambridge University Press.CS1 maint: ref=harv (link)
- Pavord, Anna (2005). The naming of names the search for order in the world of plants. New York: Bloomsbury. ISBN 9781596919655. Retrieved 18 February 2015.CS1 maint: ref=harv (link) See also ebook 2010
- Raven, Peter H.; Evert, Ray F.; Eichhorn, Susan E. (2013). Biology of plants (8th ed.). New York: W.H. Freeman. ISBN 9781464113512.
- Radosevich, Steven R.; Holt, Jodie S.; Ghersa, Claudio (1997). Weed ecology: implications for management (2nd ed.). New York: J. Wiley. ISBN 0-471-11606-8.
- Raven, Charles E. (1950) [1942]. John Ray, naturalist: his life and works (2nd ed.). Cambridge [England]: Cambridge University Press. ISBN 9780521310833. Retrieved 10 December 2015.CS1 maint: ref=harv (link)
- Reed, Barbara, ed. (2008). Plant cryopreservation a practical guide. New York: Springer. ISBN 978-0-387-72276-4.CS1 maint: ref=harv (link)
- Short, Emma; George, Alex (2013). A primer of botanical Latin with vocabulary. New York: Cambridge University Press. ISBN 9781107693753. Retrieved 14 December 2015.CS1 maint: ref=harv (link)
- Smith, Alison M; et al. (2010). Plant biology. New York, NY: Garland Science. ISBN 9780815340256. Retrieved 14 December 2015.
- Stace, Clive A. (1989) [1980]. Plant taxonomy and biosystematics (2nd. ed.). Cambridge: Cambridge University Press. ISBN 978-0-521-42785-2. Retrieved 29 April 2015.CS1 maint: ref=harv (link)
- Stebbins, G. Ledyard (1974). Flowering plants: evolution above the species level. Cambridge, Mass.: Harvard University Press. ISBN 0-674-30685-6. Retrieved 16 December 2015.CS1 maint: ref=harv (link)
- Stuessy, Tod F. (2009). Plant Taxonomy: The Systematic Evaluation of Comparative Data. Columbia University Press. ISBN 978-0-231-14712-5. Retrieved 6 February 2014.CS1 maint: ref=harv (link)
- Soltis, D.E.; Soltis, P.S.; Endress, P.K.; Chase, M.W. (2005). Phylogeny and evolution of angiosperms. Sunderland, MA: Sinauer. ISBN 9781588342010. (see also: Excerpts at Amazon
- Takhtajan, Armen Leonovich (1966). "Lilianae". Система и филогения цветкорых растений (Sistema i filogeniia tsvetkovykh rastenii) [Systema et Phylogemia Magnoliophytorum] (in Russian). trans. C Jeffrey, as Flowering plants: Origin and dispersal, Edinburgh: Oliver and Boyd, 1969. Moscow: Наука. p. 473. ISBN 0-05-001715-2. Retrieved 14 August 2015.CS1 maint: ref=harv (link)
- Takhtajan, Armen (1991). Evolutionary trends in flowering plants. New York: Columbia University Press. ISBN 9780231073288.CS1 maint: ref=harv (link)
- Takhtajan, Armen Leonovich (2009). Flowering Plants. Springer. ISBN 978-1-4020-9609-9. Retrieved 7 January 2014.CS1 maint: ref=harv (link)
- Wettstein, Richard (1924). Handbuch der Systematischen Botanik 2 vols (3rd ed.). Retrieved 15 April 2015.CS1 maint: ref=harv (link)
Symposia
- Columbus, J. T.; Friar, E. A.; Porter, J. M.; Prince, L. M.; Simpson, M. G., eds. (2006). "Symposium issue: Monocots: comparative biology and evolution (excluding Poales). Proceedings of the Third International Conference on the Comparative Biology of the Monocotyledons, 31 Mar–4 Apr 2003". Aliso. Claremont, Ca.: Rancho Santa Ana Botanic Garden. 22 (1). ISSN 0065-6275. Retrieved 18 January 2014.CS1 maint: ref=harv (link)
- Rudall, P.J.; Cribb, P.J.; Cutler, D.F.; Humphries, C.J., eds. (1995). Monocotyledons: systematics and evolution (Proceedings of the International Symposium on Monocotyledons: Systematics and Evolution, Kew 1993). Kew: Royal Botanic Gardens. ISBN 978-0-947643-85-0. Retrieved 14 January 2014.CS1 maint: ref=harv (link)
- Wilkin, Paul; Mayo, Simon J, eds. (2013). Early events in monocot evolution. Cambridge: Cambridge University Press. ISBN 978-1-107-01276-9. Retrieved 9 December 2015.CS1 maint: ref=harv (link)
- Wilson, K. L.; Morrison, D. A., eds. (2000), Monocots: Systematics and evolution (Proceedings of the Second International Conference on the Comparative Biology of the Monocotyledons, Sydney, Australia 1998), Collingwood, Australia: CSIRO, ISBN 0-643-06437-0, retrieved 14 January 2014 Excerpts
- Seberg, Ole; Petersen, Gitte; Barfod, Anders; Davis, Jerrold I., eds. (2010). Diversity, phylogeny, and evolution in the Monocotyledons: proceedings of the Fourth International Conference on the Comparative Biology of the Monocotyledons and the Fifth International Symposium on Grass Systematics and Evolution. Århus: Aarhus University Press. ISBN 978-87-7934-398-6.
- Tomlinson, P. B.; Zimmerman, Martin, eds. (1978). Tropical Trees as Living Systems (Proceedings of the fourth Cabot Symposium held at Harvard Forest, Petersham Massachusetts on April 26-30, 1976). Cambridge University Press. ISBN 978-0-521-14247-2.CS1 maint: ref=harv (link)
Chapters
- Anderson, CL; Janssen, T (2009-04-23). Monocots. pp. 203–212. ISBN 9780191560156., in Hedges & Kumar (2009)
- Chase, M. W.; Duvall, M. R.; Hills, H. G.; Conran, J. G.; Cox, A. V.; Eguiarte, L. E.; Hartwell, J.; Fay, M. F.; Caddick, L. R.; Cameron, K. M.; Hoot, S. Molecular phylogenetics of Lilianae. pp. 109–137., In Rudall et al. (1995).
- Chase, M.W.; Soltis, D. E.; Soltis, P. S.; Rudall, P. J.; Fay, M. F.; Hahn, W. H.; Sullivan, S.; Joseph, J.; Molvray, M.; Kores, P. J.; Givnish, T. J.; Sytsma, K. J.; Pires, J. C. Higher-level systematics of the monocotyledons: An assessment of current knowledge and a new classification. pp. 3–16., in Wilson & Morrison (2000)
- Chase, M. W.; Stevenson, D. W.; Wilkin, P.; Rudall, P. J. Monocot systematics: A combined analysis. 2. pp. 685–730., In Rudall et al. (1995)
- Davis, Jerrold I.; Mcneal, Joel R.; Barrett, Craig F.; Chase, Mark W.; Cohen, James I.; Duvall, Melvin R.; Givnish, Thomas J.; Graham, Sean W.; Petersen, Gitte; Pires, J. Chris; Seberg, Ole; Stevenson, Dennis W.; Leebens-Mack, Jim (2013), "Contrasting patterns of support among plastid genes and genomes for major clades of the monocotyledons", Early Events in Monocot Evolution, pp. 315–349, doi:10.1017/CBO9781139002950.015, ISBN 9781139002950, in Wilkin & Mayo (2013)
- Donoghue, Michael J.; Doyle, James A. (1989). Phylogenetic studies of seed plants and angiosperms based on morphological characters (PDF). pp. 181–193., in Fernholm, Bremer & Jörnvall (1989)
- Donoghue, Michael J.; Doyle, James A. (1989). Phylogenetic analysis of angiosperms and the relationships of Hamamelidae (PDF). pp. 17–45., In Crane & Blackmore (1989)
- Dransfield, John (2010-06-10). Growth forms of rain forest palms. pp. 247–268. ISBN 9780521142472., in Tomlinson & Zimmerman (1978)
- Givnish, T.J.; Pires, J.C.; Graham, S.W.; McPherson, M.A.; Prince, L.M.; Patterson, T.B.; Rai, H.S.; Roalson, E.R.; Evans, T.M.; Hahn, W.J; Millam, K.C.; Meerow, A.W.; Molvray, M.; Kores, P.; O'Brien, H.E.; Kress, W.J.; Hall, J.; Sytsma, K.J. Phylogeny of the monocotyledons based on the highly informative plastid gene ndhF: evidence for widespread concerted convergence (PDF). pp. 28–51. Archived from the original (PDF) on 16 January 2014. Retrieved 4 January 2014. In Columbus et al. (2006)
- Herendeen, P. S.; Crane, P. R. (1995). The fossil history of the monocotyledons. pp. 1–21.CS1 maint: ref=harv (link) In Rudall et al. (1995)
- Kubitzki, K; Rudall, PJ; Chase, MW (1998). Systematics and evolution. pp. 23–33. ISBN 9783662035337., In Kubitzki & Huber (1998).
- Panis, Bart (2008). "Cryopreservation of monocots". Plant Cryopreservation: A Practical Guide. pp. 241–280. doi:10.1007/978-0-387-72276-4_11. ISBN 978-0-387-72275-7.CS1 maint: ref=harv (link), in Reed (2008)
- Ray, John (1674). A discourse on the seeds of plants. pp. 162–169.CS1 maint: ref=harv (link), in Birch (1757)
- Rudall, Paula J.; Buzgo, Matyas (2002). "Evolutionary history of the monocot leaf". Developmental Genetics and Plant Evolution. Systematics Association Special Volumes. 20020544. pp. 431–458. doi:10.1201/9781420024982.ch23. ISBN 978-0-415-25790-9., in Cronk, Bateman & Hawkins (2002)
- Stevenson, D.W.; Loconte, H. Cladistic analysis of monocot families. pp. 543–578. in Rudall et al. (1995)
- Tillich, H.-J. (2013-06-29). Development and Organization. pp. 1–19. ISBN 9783662035337., In Kubitzki & Huber (1998)
- Tomlinson, P. B. (1995). Non-homology of vascular organisation in monocotyledons and dicotyledons. pp. 589–622.CS1 maint: ref=harv (link) In Rudall et al. (1995)
- Vines, Sydney Howard. Robert Morison 1620–1683 and John Ray 1627–1705. pp. 8–43., in Oliver (1913)
- Vogel, S (1998). Floral biology. pp. 34–48. ISBN 9783662035337., In Kubitzki & Huber (1998).
Articles
- Bentham, George (February 1877). "On the Distribution of the Monocotyledonous Orders into Primary Groups, more especially in reference to the Australian Flora, with notes on some points of Terminology". Journal of the Linnean Society of London, Botany. 15 (88): 490–520. doi:10.1111/j.1095-8339.1877.tb00261.x.CS1 maint: ref=harv (link)
- Bessey, Charles E. (1915). "The phylogenetic taxonomy of flowering plants". Annals of the Missouri Botanical Garden. 2 (1/2): 109–164. doi:10.2307/2990030. JSTOR 2990030.CS1 maint: ref=harv (link) (also at "Botanicus.org". Missouri Botanical Garden. Retrieved 5 February 2017.)
- Bremer, K. (2000). "Early Cretaceous lineages of monocot flowering plants" (PDF). Proceedings of the National Academy of Sciences USA. 97 (9): 4707–4711. Bibcode:2000PNAS...97.4707B. doi:10.1073/pnas.080421597. PMC 18297. PMID 10759567.CS1 maint: ref=harv (link)
- Bremer, K. (2002). "Gondwanan evolution of the grass alliance families (Poales)". Evolution. 56 (7): 1374–1387. doi:10.1111/j.0014-3820.2002.tb01451.x. PMID 12206239.CS1 maint: ref=harv (link)
- Bremer, Kåre; Janssen, Thomas (2006). "Gondwanan origin of major monocot groups inferred from dispersal-vicariance analysis". Aliso. 22: 22–27. doi:10.5642/aliso.20062201.03.CS1 maint: ref=harv (link)
- Cameron, K. M.; Dickison, W. C. (1998). "Foliar architecture of vanilloid orchids: Insights into the evolution of reticulate leaf venation in monocots". Bot. J. Linn. Soc. 128: 45–70. doi:10.1006/bojl.1998.0183.CS1 maint: ref=harv (link)
- Christenhusz, Maarten JM & Byng, J. W. (2016). "The number of known plants species in the world and its annual increase". Phytotaxa. Magnolia Press. 261 (3): 201–217. doi:10.11646/phytotaxa.261.3.1.CS1 maint: ref=harv (link)
- Clifford, H T (1977). "Quantitative Studies of Inter-relationships Amongst the Liliatae". Plant Syst. Evol. Suppl. 1: 77–95. doi:10.1007/978-3-7091-7076-2_6. ISBN 978-3-211-81434-5.
- Cronquist, Arthur; Takhtajan, Armen; Zimmermann, Walter (April 1966). "On the Higher Taxa of Embryobionta". Taxon. 15 (4): 129–134. doi:10.2307/1217531. JSTOR 1217531.CS1 maint: ref=harv (link)
- Cronquist, Arthur (April 1969). "Broad Features of the System of Angiosperms". Taxon. 18 (2): 188–193. doi:10.2307/1218676. JSTOR 1218676.
- Dahlgren, Gertrud (July 1989). "An updated angiosperm classification". Botanical Journal of the Linnean Society. 100 (3): 197–203. doi:10.1111/j.1095-8339.1989.tb01717.x.CS1 maint: ref=harv (link)
- Dahlgren, R. M. T. (February 1980). "A revised system of classification of the angiosperms". Botanical Journal of the Linnean Society. 80 (2): 91–124. doi:10.1111/j.1095-8339.1980.tb01661.x.CS1 maint: ref=harv (link)
- Dahlgren, Rolf; Rasmussen, Finn N. (1983). "Monocotyledon Evolution: Characters and Phylogenetic Estimation". Evolutionary Biology. 16: 255–395. doi:10.1007/978-1-4615-6971-8_7.
- Donoghue, Michael J. (2005). "Key innovations, convergence, and success: macroevolutionary lessons from plant phylogeny" (PDF). Paleobiology. 31: 77–93. doi:10.1666/0094-8373(2005)031[0077:KICASM]2.0.CO;2.CS1 maint: ref=harv (link)
- Doyle, James A; Donoghue, Michael J (April–June 1992). "Fossils and seed plant phylogeny reanalyzed" (PDF). Brittonia. 44 (2): 89–106. doi:10.2307/2806826. JSTOR 2806826.
- Fay, Michael F. (May 2013). "Monocots". Botanical Journal of the Linnean Society. 172 (1): 1–4. doi:10.1111/boj.12052.CS1 maint: ref=harv (link)
- Friis, E. M.; Pedersen, K. R.; Crane, P. R. (2004). "Araceae from the early Cretaceous of Portugal: Evidence on the emergence of monocotyledons". Proceedings of the National Academy of Sciences. 101 (47): 16565–16570. Bibcode:2004PNAS..10116565F. doi:10.1073/pnas.0407174101. PMC 534535. PMID 15546982.CS1 maint: ref=harv (link)
- Friis, E. M.; Pedersen, K. R.; Crane, P. R. (2006). "Cretaceous angiosperm flowers: innovation and evolution in plant reproduction". Palaeogeog. Palaeoclim. Palaeoecol. 232 (2–4): 251–293. Bibcode:2006PPP...232..251F. doi:10.1016/j.palaeo.2005.07.006.CS1 maint: ref=harv (link)
- Gandolfo, M. A; Nixon, K. C.; Crepet, W. L.; Stevenson, D. W.; Friis, E. M. (6 August 1998). "Oldest known fossils of monocotyledons". Nature. 394 (6693): 532–533. Bibcode:1998Natur.394..532G. doi:10.1038/28974.
- Gandolfo, M. A.; Nixon, K. C.; Crepet, W. L. (2002). "Triuridaceae fossil flowers from the Upper Cretaceous of New Jersey". American Journal of Botany. 89 (12): 1940–1957. doi:10.3732/ajb.89.12.1940. PMID 21665623.CS1 maint: ref=harv (link)
- Hallier, Hans (31 July 1905). "Provisional scheme of the natural (phylogenetic) system of the flowering plants". New Phytologist. 4 (7): 151–162. doi:10.1111/j.1469-8137.1905.tb05894.x. hdl:2027/hvd.32044107266454.CS1 maint: ref=harv (link)
- Henslow, George (May 1893). "A Theoretical Origin of Endogens from Exogens, through Self-Adaptation to an Aquatic Habit". Botanical Journal of the Linnean Society. 29 (204): 485–528. doi:10.1111/j.1095-8339.1893.tb02273.x.CS1 maint: ref=harv (link)
- Herendeen, Patrick S.; Crane, Peter R.; Drinnan, Andrew N. (January 1995). "Fagaceous flowers, fruits, and cupules from the Campanian (Late Cretaceous) of Central Georgia, USA". International Journal of Plant Sciences. 156 (1): 93–116. doi:10.1086/297231. JSTOR 2474901.CS1 maint: ref=harv (link)
- Hertweck, Kate L.; Kinney, Michael S.; Stuart, Stephanie A.; Maurin, Olivier; Mathews, Sarah; Chase, Mark W.; Gandolfo, Maria A.; Pires, J. Chris (July 2015), "Phylogenetics, divergence times and diversification from three genomic partitions in monocots", Botanical Journal of the Linnean Society, 178 (3): 375–393, doi:10.1111/boj.12260
- Huber, H (1969). "Die Samenmerkmale und Verwandtschaftsverhältnisse der Liliiflorae". Mitt. Bot. Staatssamml.[Mitteilungen der Botanischen Staatssammlung München] (in German). 8: 219–538. Retrieved 10 February 2015.CS1 maint: ref=harv (link)
- Moore, John P.; Lindsey, George G.; Farrant, Jill M.; Brandt, Wolf F. (2007). "An Overview of the Biology of the Desiccation-tolerant Resurrection Plant Myrothamnus flabellifolia" (PDF). Annals of Botany. 99 (2): 211–217. doi:10.1093/aob/mcl269. PMC 2803006. PMID 17218343. Retrieved November 3, 2015.
- Petit, G.; DeClerck, F. A. J.; Carrer, M.; Anfodillo, T. (31 January 2014). "Axial vessel widening in arborescent monocots". Tree Physiology. 34 (2): 137–145. doi:10.1093/treephys/tpt118. PMID 24488857.
- Sanderson, Michael J. (1997). "A nonparametric approach to estimating divergence times in the absence of rate constancy" (PDF). Molecular Biology and Evolution. 14 (12): 1218–1231. doi:10.1093/oxfordjournals.molbev.a025731.CS1 maint: ref=harv (link)
- Sanderson, M. J.; Thorne, J. L.; Wikström, N.; Bremer, K. (2004). "Molecular evidence on plant divergence times". American Journal of Botany. 91 (10): 1656–1665. doi:10.3732/ajb.91.10.1656. PMID 21652315.
- Strong, Donald R.; Ray, Thomas S. (1 January 1975). "Host Tree Location Behavior of a Tropical Vine (Monstera gigantea) by Skototropism". Science. 190 (4216): 804–806. Bibcode:1975Sci...190..804S. doi:10.1126/science.190.4216.804. JSTOR 1741614.CS1 maint: ref=harv (link)
- Takhtajan, A. (June 1964). "The Taxa of the Higher Plants above the Rank of Order". Taxon. 13 (5): 160–164. doi:10.2307/1216134. JSTOR 1216134.CS1 maint: ref=harv (link)
- Tang, Cuong Q.; Orme, C. David L.; Bunnefeld, Lynsey; Jones, F. Andrew; Powell, Silvana; Chase, Mark W.; Barraclough, Timothy G.; Savolainen, Vincent (October 2016). "Global monocot diversification: geography explains variation in species richness better than environment or biology". Botanical Journal of the Linnean Society. doi:10.1111/boj.12497.
- Thorne, Robert F. (1976). "A phylogenetic classification of the Angiospermae". Evolutionary Biology. 9: 35–106. doi:10.1007/978-1-4615-6950-3_2. ISBN 978-1-4615-6952-7.CS1 maint: ref=harv (link)
- Thorne, R. F. (1992a). "Classification and geography of the flowering plants". The Botanical Review. 58 (3): 225–348. doi:10.1007/BF02858611.CS1 maint: ref=harv (link)
- Thorne, R. F. (1992b). "An updated phylogenetic classification of the flowering plants". Aliso. 13 (2): 365–389. doi:10.5642/aliso.19921302.08.CS1 maint: ref=harv (link)
- Tomlinson, P. B. (1970). "Monocotyledons - towards an understanding of their morphology and anatomy". Adv. Bot. Res. Advances in Botanical Research. 3: 207–292. doi:10.1016/S0065-2296(08)60321-3. ISBN 9780120059034.CS1 maint: ref=harv (link)
- Tomlinson, P. B.; Esler, A. E. (1 December 1973). "Establishment growth in woody monocotyledons native to New Zealand". New Zealand Journal of Botany. 11 (4): 627–644. doi:10.1080/0028825X.1973.10430305.CS1 maint: ref=harv (link)
- Wikström, Niklas; Savolainen, Vincent; Chase, Mark W. (2001). "Evolution of the angiosperms: calibrating the family tree". Proceedings of the Royal Society of London B. 268 (1482): 2211–2220. doi:10.1098/rspb.2001.1782. PMC 1088868. PMID 11674868.CS1 maint: ref=harv (link)
- Zimmermann, Martin H.; Tomlinson, P. B. (June 1972). "The vascular system of monocotyledonous stems". Botanical Gazette. 133 (2): 141–155. doi:10.1086/336628.CS1 maint: ref=harv (link)
Phylogenetics
- Bremer, Kåre; Wanntorp, Hans-Erik (Aug 1978). "Phylogenetic Systematics in Botany". Taxon. 27 (4): 317–329. doi:10.2307/1220367. JSTOR 1220367.CS1 maint: ref=harv (link)
- Cantino, Philip D.; Doyle, James A.; Graham, Sean W.; Judd, Walter S.; Olmstead, Richard G.; Soltis, Douglas E.; Soltis, Pamela S.; Donoghue, Michael J. (2007). "Towards a phylogenetic nomenclature of Tracheophyta" (PDF). Taxon. 56 (3): 822–846. doi:10.2307/25065865. JSTOR 25065865.
- Chase, Mark W.; Soltis, Douglas E.; Olmstead, Richard G.; Morgan, David; Les, Donald H.; Mishler, Brent D.; Duvall, Melvin R.; Price, Robert A.; Hills, Harold G.; Qiu, Yin-Long; Kron, Kathleen A.; Rettig, Jeffrey H.; Conti, Elena; Palmer, Jeffrey D.; Manhart, James R.; Sytsma, Kenneth J.; Michaels, Helen J.; Kress, W. John; Karol, Kenneth G.; Clark, W. Dennis; Hedren, Mikael; Gaut, Brandon S.; Jansen, Robert K.; Kim, Ki-Joong; Wimpee, Charles F.; Smith, James F.; Furnier, Glenn R.; Strauss, Steven H.; Xiang, Qui-Yun; Plunkett, Gregory M.; Soltis, Pamela S.; Swensen, Susan M.; Williams, Stephen E.; Gadek, Paul A.; Quinn, Christopher J.; Eguiarte, Luis E.; Golenberg, Edward; Learn, Gerald H.; Graham, Sean W.; Barrett, Spencer C. H.; Dayanandan, Selvadurai; Albert, Victor A. (1993). "Phylogenetics of Seed Plants: An Analysis of Nucleotide Sequences from the Plastid Gene rbcL" (PDF). Annals of the Missouri Botanical Garden. 80 (3): 528. doi:10.2307/2399846. JSTOR 2399846.
- Chase, Mark W. (2004). "Monocot relationships: an overview". American Journal of Botany. 91 (10): 1645–1655. doi:10.3732/ajb.91.10.1645. PMID 21652314.CS1 maint: ref=harv (link)
- Davis, Jerrold I.; Stevenson, Dennis W.; Petersen, Gitte; Seberg, Ole; Campbell, Lisa M.; Freudenstein, John V.; Goldman, Douglas H.; Hardy, Christopher R.; Michelangeli, Fabian A.; Simmons, Mark P.; Specht, Chelsea D.; Vergara-Silva, Francisco; Gandolfo, María (1 July 2004). "A Phylogeny of the Monocots, as Inferred from rbcL and atpA Sequence Variation, and a Comparison of Methods for Calculating Jackknife and Bootstrap Values" (PDF). Systematic Botany. 29 (3): 467–510. doi:10.1600/0363644041744365.
- Du, Zhi-Yuan; Wang, Qing-Feng (July 2016). "Phylogenetic tree of vascular plants reveals the origins of aquatic angiosperms". Journal of Systematics and Evolution. 54 (4): 342–348. doi:10.1111/jse.12182.
- Duvall, Melvin R.; Clegg, Michael T.; Chase, Mark W.; Clark, W. Dennis; Kress, W. John; Hills, Harold G.; Eguiarte, Luis E.; Smith, James F.; Gaut, Brandon S.; Zimmer, Elizabeth A.; Learn, Gerald H. (1 January 1993). "Phylogenetic Hypotheses for the Monocotyledons Constructed from rbcL Sequence Data". Annals of the Missouri Botanical Garden. 80 (3): 607–619. doi:10.2307/2399849. JSTOR 2399849.
- Endress, P. K.; Doyle, J. A. (8 January 2009). "Reconstructing the ancestral angiosperm flower and its initial specializations". American Journal of Botany. 96 (1): 22–66. doi:10.3732/ajb.0800047. PMID 21628175.
- Givnish, Thomas J.; Pires, J.Chris; Graham, Sean W.; McPherson, Marc A.; Prince, Linda M.; Patterson, Thomas B.; Rai, Hardeep S.; Roalson, Eric H.; Evans, Timothy M.; Hahn, William J; Millam, Kendra C.; Meerow, Alan W; Molvray, Mia; Kores, Paul J.; O'Brien, Heath E.; Hall, Jocelyn C.; Kress, W. John; Sytsma, Kenneth J. (2005). "Repeated evolution of net venation and fleshy fruits among monocots in shaded habitats confirms a priori predictions: evidence from an ndhF phylogeny". Proceedings of the Royal Society B: Biological Sciences. 272 (1571): 1481–1490. doi:10.1098/rspb.2005.3067. PMC 1559828. PMID 16011923.
- Givnish, Thomas J.; Ames, Mercedes; McNeal, Joel R.; McKain, Michael R.; Steele, P. Roxanne; dePamphilis, Claude W.; Graham, Sean W.; Pires, J. Chris; Stevenson, Dennis W.; Zomlefer, Wendy B.; Briggs, Barbara G.; Duvall, Melvin R.; Moore, Michael J.; Heaney, J. Michael; Soltis, Douglas E.; Soltis, Pamela S.; Thiele, Kevin; Leebens-Mack, James H. (27 December 2010). "Assembling the Tree of the Monocotyledons: Plastome Sequence Phylogeny and Evolution of Poales". Annals of the Missouri Botanical Garden. 97 (4): 584–616. doi:10.3417/2010023.
- Givnish, Thomas J.; Zuluaga, Alejandro; Spalink, Daniel; Soto Gomez, Marybel; Lam, Vivienne K. Y.; Saarela, Jeffrey M.; Sass, Chodon; Iles, William J. D.; de Sousa, Danilo José Lima; Leebens-Mack, James; Chris Pires, J.; Zomlefer, Wendy B.; Gandolfo, Maria A.; Davis, Jerrold I.; Stevenson, Dennis W.; dePamphilis, Claude; Specht, Chelsea D.; Graham, Sean W.; Barrett, Craig F.; Ané, Cécile (November 2018). "Monocot plastid phylogenomics, timeline, net rates of species diversification, the power of multi-gene analyses, and a functional model for the origin of monocots". American Journal of Botany. 105 (11): 1888–1910. doi:10.1002/ajb2.1178. PMID 30368769.
- Goremykin, Vadim V.; Hansman, Sabine; Martin, William F. (March 1997). "Evolutionary analysis of 58 proteins encoded in six completely sequenced chloroplast genomes: revised molecular estimates of two seed plant divergence times". Plant Syst. Evol. 206 (1): 337–351. doi:10.1007/bf00987956.CS1 maint: ref=harv (link)
- Hertweck, Kate L.; Kinney, Michael S.; Stuart, Stephanie A.; Maurin, Olivier; Mathews, Sarah; Chase, Mark W.; Gandolfo, Maria A.; Pires, J. Chris (July 2015). "Phylogenetics, divergence times and diversification from three genomic partitions in monocots". Botanical Journal of the Linnean Society. 178 (3): 375–393. doi:10.1111/boj.12260.
- Janssen, Thomas; Bremer, Kare (December 2004). "The age of major monocot groups inferred from 800+ rbcL sequences". Botanical Journal of the Linnean Society. 146 (4): 385–398. doi:10.1111/j.1095-8339.2004.00345.x.CS1 maint: ref=harv (link)
- Leebens-Mack, Jim; Raubeson, Linda A.; Cui, Liying; Kuehl, Jennifer V.; Fourcade, Mathew H.; Chumley, Timothy W.; Boore, Jeffrey L.; Jansen, Robert K.; dePamphilis, Claude W. (October 2005). "Identifying the basal angiosperm node in chloroplast genome phylogenies: Sampling one's way out of the Felsenstein zone". Mol. Biol. Evol. 22 (10): 1948–1963. doi:10.1093/molbev/msi191. PMID 15944438.
- Loconte, Henry; Stevenson, Dennis W. (September 1991). "Cladistics of the Magnoliidae". Cladistics. 7 (3): 267–296. doi:10.1111/j.1096-0031.1991.tb00038.x.CS1 maint: ref=harv (link)
- Patterson, T. B.; Givnish, T. J. (2002). "Phylogeny, concerted convergence, and phylogenetic niche conservatism in the core Liliales: insights from rbcL and ndhF sequence data" (PDF). Evolution. 56 (2): 233–252. doi:10.1111/j.0014-3820.2002.tb01334.x. PMID 11926492. Archived from the original on April 21, 2004. Retrieved 14 January 2014.CS1 maint: ref=harv (link) CS1 maint: unfit url (link)
- Qiu, Yin-Long; Li, Libo; Wang, Bin; Xue, Jia-Yu; Hendry, Tory A.; Li, Rui-Qi; Brown, Joseph W.; Liu, Yang; Hudson, Geordan T.; Chen, Zhi-Duan (November 2010). "Angiosperm phylogeny inferred from sequences of four mitochondrial genes". Journal of Systematics and Evolution. 48 (6): 391–425. doi:10.1111/j.1759-6831.2010.00097.x.
- Savard, L.; Strauss, S. H.; Chase, M. W.; Michaud, M.; Bosquet, J. (May 1994). "Chloroplast and nuclear gene sequences indicate late Pennsylvanian time for the last common ancestor of extant seed plants". Proceedings of the National Academy of Sciences of the United States of America. 91 (11): 5163–5167. Bibcode:1994PNAS...91.5163S. doi:10.1073/pnas.91.11.5163. PMC 43952. PMID 8197201.
- Soltis, Pamela S; Soltis, Douglas E (2004). "The origin and diversification of angiosperms". American Journal of Botany. 91 (10): 1614–1626. doi:10.3732/ajb.91.10.1614. PMID 21652312.CS1 maint: ref=harv (link)
- Soltis, D. E.; Smith, S. A.; Cellinese, N.; Wurdack, K. J.; Tank, D. C.; Brockington, S. F.; Refulio-Rodriguez, N. F.; Walker, J. B.; Moore, M. J.; Carlsward, B. S.; Bell, C. D.; Latvis, M.; Crawley, S.; Black, C.; Diouf, D.; Xi, Z.; Rushworth, C. A.; Gitzendanner, M. A.; Sytsma, K. J.; Qiu, Y.-L.; Hilu, K. W.; Davis, C. C.; Sanderson, M. J.; Beaman, R. S.; Olmstead, R. G.; Judd, W. S.; Donoghue, M. J.; Soltis, P. S. (8 April 2011). "Angiosperm phylogeny: 17 genes, 640 taxa". American Journal of Botany. 98 (4): 704–730. doi:10.3732/ajb.1000404. PMID 21613169.
- Soltis, Pamela S; Soltis, Douglas E (April 2016). "Ancient WGD events as drivers of key innovations in angiosperms". Current Opinion in Plant Biology. 30: 159–165. doi:10.1016/j.pbi.2016.03.015. PMID 27064530.CS1 maint: ref=harv (link)
- Trias-Blasi, Anna; Baker, William J.; Haigh, Anna L.; Simpson, David A.; Weber, Odile; Wilkin, Paul (25 June 2015). "A genus-level phylogenetic linear sequence of monocots". Taxon. 64 (3): 552–581. doi:10.12705/643.9.
- Zeng, Liping; Zhang, Qiang; Sun, Renran; Kong, Hongzhi; Zhang, Ning; Ma, Hong (24 September 2014). "Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times". Nature Communications. 5 (4956): 4956. Bibcode:2014NatCo...5.4956Z. doi:10.1038/ncomms5956. PMC 4200517. PMID 25249442.
APG
- APG (1998). "An ordinal classification for the families of flowering plants". Annals of the Missouri Botanical Garden. 85 (4): 531–553. doi:10.2307/2992015. JSTOR 2992015.CS1 maint: ref=harv (link)
- APG II (2003). "An Update of the Angiosperm Phylogeny Group Classification for the orders and families of flowering plants: APG II". Botanical Journal of the Linnean Society. 141 (4): 399–436. doi:10.1046/j.1095-8339.2003.t01-1-00158.x.CS1 maint: ref=harv (link)
- APG III (2009). "An Update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III". Botanical Journal of the Linnean Society. 161 (2): 105–121. doi:10.1111/j.1095-8339.2009.00996.x. Archived from the original on 25 May 2017. Retrieved 3 January 2014.CS1 maint: ref=harv (link)
- APG IV (2016). "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV". Botanical Journal of the Linnean Society. 181 (1): 1–20. doi:10.1111/boj.12385.CS1 maint: ref=harv (link)
- Chase, Mark W; Reveal, James L (2009). "A phylogenetic classification of the land plants to accompany APG III" (PDF). Botanical Journal of the Linnean Society. 161 (2): 122–127. doi:10.1111/j.1095-8339.2009.01002.x. Retrieved 21 April 2015.CS1 maint: ref=harv (link)
- Haston, Elspeth; Richardson, James E.; Stevens, Peter F.; Chase, Mark W.; Harris, David J. (2009). "The Linear Angiosperm Phylogeny Group (LAPG) III: a linear sequence of the families in APG III". Botanical Journal of the Linnean Society. 161 (2): 128–131. doi:10.1111/j.1095-8339.2009.01000.x.
Websites and databases
- Hahn, William J. (1997). "Monocotyledons". Tree of Life web project. Retrieved 6 February 2017.
- Stevens, P.F. (2015) [2001], Angiosperm Phylogeny Website, Missouri Botanical Garden, retrieved 31 January 2017CS1 maint: ref=harv (link) (see also Angiosperm Phylogeny Website)
- Givnish, Thomas. "Assembling the phylogeny of the monocots". Monocot AToL Project. Madison: Department of Botany, University of Wisconsin. Retrieved 1 March 2017.
- CoL (2015). "Catalogue of Life". ITIS. Retrieved 6 February 2017.CS1 maint: ref=harv (link)
- IUCN (2016). "The IUCN Red List of Threatened Species". International Union for Conservation of Nature and Natural Resources. Retrieved 6 February 2017.
- "National Botanic Gardens of Ireland". 2016. Retrieved 19 January 2016.
- "Tropicos". Missouri Botanical Garden. 2015. Retrieved 30 December 2015.
- "Class: Monocotyledoneae - Monocot". Plant Life Forms. Retrieved 7 February 2017.
External links
| Wikispecies has information related to Monocots |
| Wikimedia Commons has media related to monocots. |
