Liquid carbon dioxide
Liquid carbon dioxide is the liquid state of carbon dioxide, which cannot occur under atmospheric pressure. It can only exist at a pressure above 5.1 atm (5.2 bar; 75 psi), under 31.1 °C (temperature of critical point) and above -56.6 °C (temperature of triple point). Its uses and applications include the extraction of virgin olive oil paste, fire extinguishers, and as a coolant.[1]
| Liquid carbon dioxide | |
|---|---|
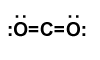 | |
| Examples | CO2 |
| Point group | D∞h |
| Coordination number | 2 |
| Bond angle(s) | 180° |
| μ (Polarity) | non-polar |
Properties
General properties
Liquid carbon dioxide is a type of liquid which is formed from highly compressed and cooled gaseous carbon dioxide. It does not form under atmospheric conditions. It only exists when the pressure is above 5.1 atm and the temperature is under 31.1 °C (temperature of critical point) and above -56.6 °C (temperature of triple point). The chemical symbol remains the same as gaseous carbon dioxide (CO₂).[2] It is transparent and odorless and the density of it is 1101 kg/m³ when the liquid is at full saturation at -37 °C.[3][4]
The solubility of water in liquid carbon dioxide is measured in a range of temperatures ranging from -29°C to 22.6 °C. At this temperature, the pressure is measured in a range from 15 to 50 atmospheres. [5]
Viscosity of liquid carbon dioxide
Introduction
The viscosity is one of the measures of a fluid substance that is the resistance of itself. It can be described as high viscosity or low viscosity. Before the study of the viscosity of a liquid, a scientist called van der Gulik has explained how the viscosity of gas determines. He said that the increase in temperature can increase the viscosity of the gas, but it is opposite to liquid. He also proposed the influence of the molecules exerts on each other to viscosity. Another person called Brillouin also has mentioned that the concept of free path of molecules should be given up.[6] After several ideas have been mentioned and proposed, the typical image of the viscosity of liquids is that the molecule is actively bouncing in a space that is formed by other molecules when the momentum has been exchanged as collisions. Therefore, the viscosity coefficient is measured by the efficiency of exchange and by the number of collisions per unit of time and volume.[6]
Method
The viscosity of liquid carbon dioxide is measured by using a type of viscometer called vibrating-wire viscometer at temperatures of five levels 220K, 230K, 240K, 260K and 280K that are along isotherms between 217K (triple point temperature) and 304K (critical point temperature) over the complete liquid range.[6] The accuracy of this measurement is estimated to 0.01. By using the vibrating-wire viscometer that including a wire, there is an alternating electrical current generates the Lorentz force and an electromagnet raises a magnetic field. After the electrical current stopping, an induction voltage is caused by the free damped oscillation of the wire when it has been in the magnetic field caused by the electromagnet. The induction voltage has been enlarged, sampled and digitized and it is kept as a set of 2048 numbers on a computer disk and the data of this signal is a measure of the sample liquid for its viscosity.[7]
Conclusion
When finished the common cleaning and filling process, the vessel should be at approximately 8 MPa after it has been set in a wanted and suitable temperature. However, before the above condition, the vessel needs to be pressurized at room temperature firstly. As a result, it has avoided the leakage of the liquid carbon dioxide sample and prevented the extra damping because of the insufficient equilibrium.[6] The next step is needed to satisfy the equilibrium. Under these circumstances, the first measurement is used to determine the resonant frequency of the wire. The synthesizer delivers the electrical current that is adjusted to this frequency and measures it 5 to 7 times with a various periods of time. The resulting series of 2048 numbers need to transfer following Fourier transformation. Hence, the width and the resonance frequency are determined by using the computed signal to match with the Lorentz function. The resonant frequency is in a range of 765 Hz at 280K,350MPa to 823 Hz at 220K, 0.55MPa. The scientists have set several tables with various temperature to show the viscosity of liquid carbon dioxide.[7] Generally, at a certain temperature, the viscosity of liquid carbon dioxide will increase, as the pressure is increasing. For example, at the temperature of 220.005 K, the range of viscosity is from 243.4μPa s at 0.559 MPa to 290.3μPa s at 22.53 MPa.[7]
Related history
Uses
Reduction of emission of carbon dioxide
High-purity liquefied carbon dioxide plant
It is a type of plant that tries to reduce the emission of carbon dioxide by sealing up for safekeeping underground. The plant will use the emission of carbon dioxide gas as the raw material from other factories. The emission gas may come from thermal power factories, ammonia manufacturing factories, petrochemical refining and so on. The "raw material" will be produced to the high quality carbon dioxide after purifying, compressing, dehumidifying and liquefying. The facility is very big with a capacity of 7,200 m³N/h for processing and after expansion, the capacity has changed to 9,000 m³N/h.[9] The production of liquid carbon dioxide from this plant can be used for solid phase of carbon dioxide and can be used in producing carbonated beverages.
Key features
There are four important advantages of this high-purity liquefied carbon dioxide plant.[9] Firstly, it can produce high purity of liquid carbon dioxide that reaches at least 99.995 Vol%. Secondly, the requirement of unit power is low and it is an energy efficient facility. Thirdly, it may shorten the processing time by the process of skidding and it can save the occupied space. Lastly, it can adjust when the volume of gas production due to its automatic facility.[9]
Carbon capture and storage
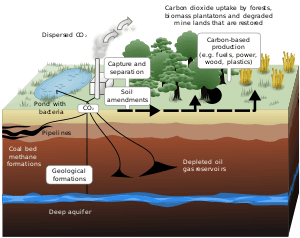
CCS is an important technology to help to reduce carbon dioxide emission. In this application, liquid carbon dioxide is the majority of the inventory.[10]
Liquid carbon dioxide has no corrosion due to that there is no free water or impurities in it. The key point of CCS application is dissolving water in liquid carbon dioxide rather than dissolving carbon dioxide in water. The reason is that there is no loosely oxygen atom which will form oxides. Hence, the potential of corrosion does not exist. Besides, considering carefully the formation of hydrate when the application happens at low temperature as there may be a corrosive potential of liquid carbon dioxide. The formation of hydrates depends on temperature, pressure, the gas pollution in carbon dioxides and the concentration of water. In the CCS system, all of the water can be dissolved in liquid carbon dioxide at a range of 10 °C to 25 °C, but when the temperature is under an equilibrium temperature(4 °C), it is possible that the corrosion will appear due to the hydrates has formed at some level of water content.[11]
Extraction of extra virgin olive paste
Extra virgin olive oil (EVOO) is a type of olive oil that extracted from the fruit of Olea Europeae. To obtain further oil of high quality from the leftover olive paste, solvent extraction can be used, the application of compressed fluids has been experimented, like liquid carbon dioxide.
Firstly, the collecting of samples that are extracted from the olive paste by liquid carbon dioxide. It is performed as two different ratios of solid-liquid, one is 1:5 and another one is 1:10 and two different periods for calculating the yield of olive oil, one is every 30 minutes and another one is after 180 minutes. Therefore, there are three sets of experiments where the difference between ratio and time can be tested together. And the tests within every 30 minutes are called discontinuous process, another one within after 180 minutes is called continuous process.[12]
After finishing each of the tests, the yield of olive oil extraction can be shown by using a diagram. The diagram has been shown with time (min) at x-axis and the yield of extraction on the y-axis and there are two lines one is with the solid-liquid ratio of 1 to 5, another one is with the solid-liquid ratio of 1 to 5. It is clear that the discontinuous process has extracted a higher yield. The yield is 15.49% and 16.64% of the continuous extraction after 180 minutes, for 1:5 and 1:10, respectively. Compared with other classical physical applications, the advantages of extracting olive oil within liquid carbon dioxide include the limit hydrolysis and oxidation reaction, the concentration of bisphenols is higher than classic methods, the presence of limonene and other aromatic compounds.[12] The most important one is that there is no large amount of pollution requiring disposal due to the production of olive mill wastewater. Hence, the liquid phase of liquid carbon dioxide.
Others

The other uses of liquid carbon dioxide include the preservation of food, fire extinguisher, softening drinks and even the entertainment industry. For food preservation, the food industry is largely using liquid carbon dioxide to refrigerate, preserve, store and soften. For fire extinguisher, as the liquid carbon dioxide is anti-flammable.[2]Besides, the use of liquid carbon dioxide also cools the burning surface to avoid further damage. For softening drinks, the liquid carbon dioxide is compressed by gas carbon dioxide under certain conditions. It has been largely used in the production of the soft drink. Similarly, to remove caffeine from coffee, the liquid carbon oxide can also perform usefully as it is a flexible solvent.[2]For the entertainment industry, the liquid carbon dioxide can be used for haze effects and fog effects. The haze effect is about television production, the liquid carbon dioxide is used for creating homogeneous cloud effects that are used to reveal the lights in music concerts. The fog effect is an application in movie scenes, the liquid carbon dioxide can produce low-lying fog within fog machines (theatrical smoke and fog).
See also
As the liquid carbon dioxide is the liquid state of carbon dioxide, some other chemical compounds and elements may have a liquid state.
References
- "How to Make Liquid CO2". Sciencing. Retrieved 2019-06-11.
- "What is Liquid CO2 and What Can It Be Useful For? | Co2 Gas Blog". Co2 Gas Company. 2017-10-08. Retrieved 2019-04-30.
- "Carbon dioxide", Wikipedia, 2019-04-26, retrieved 2019-04-30
- "Carbon dioxide", Wikipedia, 2019-05-20, retrieved 2019-05-21
- Stone, Hosmer W. (December 1943). "Solubility of Water in Liquid Carbon Dioxide". Industrial & Engineering Chemistry. 35 (12): 1284–1286. doi:10.1021/ie50408a015. ISSN 0019-7866.
- van der Gulik, P. S.; El Kharraz, M. (1995-01-01). "The viscosity of liquid carbon dioxide" (PDF). International Journal of Thermophysics. 16 (1): 145–153. Bibcode:1995IJT....16..145V. doi:10.1007/BF01438965. ISSN 1572-9567.
- Van Der Gulik, P.S. (1997). "Viscosity of carbon dioxide in the liquid phase". Physica A: Statistical Mechanics and Its Applications. 238 (1–4): 81–112. Bibcode:1997PhyA..238...81V. doi:10.1016/S0378-4371(96)00466-9.
- Gupta, _; Bobier, _ (1998). "The History and Success of Liquid CO2 and CO2/N2 Fracturing System". SPE Gas Technology Symposium. Society of Petroleum Engineers. doi:10.2118/40016-ms.CS1 maint: numeric names: authors list (link)
- "High-purity liquefied carbon dioxide plants : Industrial plant : Hitachi". www.hitachi.com. Retrieved 2019-05-28.
- "Carbon capture and storage", Wikipedia, 2019-05-16, retrieved 2019-05-21
- "2.2 Liquid carbon dioxide | Global CCS Institute". hub.globalccsinstitute.com. Retrieved 2019-05-04.
- Romano, Raffaele; Manzo, Nadia; Montefusco, Immacolata; Romano, Annalisa; Santini, Antonello (2014-03-12). "Liquid Carbon Dioxide Use in the Extraction of Extra Virgin Olive Oil From Olive Paste". Journal of Food Research. 3 (4): 119. doi:10.5539/jfr.v3n4p119. ISSN 1927-0895.