Hashimoto's thyroiditis
Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis and Hashimoto's disease, is an autoimmune disease in which the thyroid gland is gradually destroyed.[1][6] Early on there may be no symptoms.[1] Over time the thyroid may enlarge, forming a painless goiter.[1] Some people eventually develop hypothyroidism with accompanying weight gain, feeling tired, constipation, depression, and general pains.[1] After many years the thyroid typically shrinks in size.[1] Potential complications include thyroid lymphoma.[2]
| Hashimoto's thyroiditis | |
|---|---|
| Other names | Chronic lymphocytic thyroiditis, autoimmune thyroiditis, struma lymphomatosa, Hashimoto's disease |
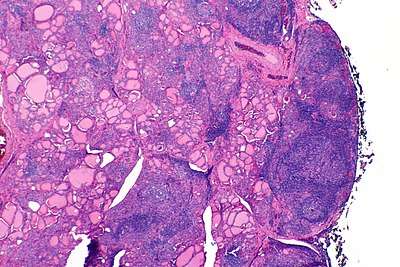 | |
| The thyroid of someone with Hashimoto's thyroiditis as seen with a microscope at low magnification | |
| Specialty | Endocrinology |
| Symptoms | Painless goiter, weight gain, feeling tired, constipation, depression[1] |
| Complications | Thyroid lymphoma.[2] |
| Usual onset | 30–50 years old[1][3] |
| Causes | Genetic and environmental factors.[4] |
| Risk factors | Family history, another autoimmune disease[1] |
| Diagnostic method | TSH, T4, anti-thyroid autoantibodies[1] |
| Differential diagnosis | Graves’ disease, nontoxic nodular goiter[5] |
| Treatment | Levothyroxine, surgery[1][5] |
| Frequency | 5% at some point[4] |
Hashimoto's thyroiditis is thought to be due to a combination of genetic and environmental factors.[4] Risk factors include a family history of the condition and having another autoimmune disease.[1] Diagnosis is confirmed with blood tests for TSH, T4, and anti-thyroid autoantibodies.[1] Other conditions that can produce similar symptoms include Graves’ disease and nontoxic nodular goiter.[5]
Hashimoto's thyroiditis is typically treated with levothyroxine.[1][7] If hypothyroidism is not present, some may recommend no treatment, while others may treat to try to reduce the size of the goiter.[1][8] Those affected should avoid eating large amounts of iodine; however, sufficient iodine is required especially during pregnancy.[1] Surgery is rarely required to treat the goiter.[5]
Hashimoto's thyroiditis affects about 5% of Caucasians at some point in their life.[4] It typically begins between the ages of 30 and 50 and is much more common in women than men.[1][3] Rates of the disease appear to be increasing.[5] It was first described by the Japanese physician Hakaru Hashimoto in 1912.[9] In 1957 it was recognized as an autoimmune disorder.[10]
Signs and symptoms
There are many symptoms that are attributed to Hashimoto's thyroiditis or Hashimoto's disease. The most common symptoms include the following: fatigue, weight gain, pale or puffy face, feeling cold, joint and muscle pain, constipation, dry and thinning hair, heavy menstrual flow or irregular periods, depression, panic disorder, a slowed heart rate, and problems getting pregnant and maintaining pregnancy.[11]
Hashimoto’s disease is about seven times more common in women than in men. It can occur in teens and young women, but more commonly appears in middle age, particularly for men. People who develop Hashimoto’s disease often have family members who have thyroid or other autoimmune diseases, and sometimes have other autoimmune diseases themselves.[12]
The thyroid gland may become firm, large, and lobulated in Hashimoto's thyroiditis, but changes in the thyroid can also be nonpalpable.[13] Enlargement of the thyroid is due to lymphocytic infiltration and fibrosis, rather than tissue hypertrophy. While their role in the initial destruction of the follicles is unclear, antibodies against thyroid peroxidase (TPO) (also called TPOAb) or thyroglobulin are relevant, as they serve as markers for detecting the disease and its severity.[14] They are hypothesized to be the secondary products of the T cell mediated destruction of the gland.[15]
It is also characterized by invasion of the thyroid tissue by leukocytes, mainly T-lymphocytes. A rare but serious complication is thyroid lymphoma, generally the B-cell type, non-Hodgkin lymphoma.[16]
Risk factors
The strong genetic component is borne out in studies on monozygotic twins, with a concordance of 38–55%, with an even higher concordance of circulating thyroid antibodies not in relation to clinical presentation (up to 80% in monozygotic twins). Neither result was seen to a similar degree in dizygotic twins, offering strong favour for high genetic aetiology.[17]
Hashimoto's thyroiditis is associated with CTLA-4 (Cytotoxic T-lymphocyte Antigen-4) gene polymorphisms. CTLA-4 downregulates., i.e. transmits an inhibitory signal to T cells, so reduced functioning is associated with increased T-lymphocyte activity.[18][19] A family history of thyroid disorders is common, with the HLA-DR5 gene most strongly implicated conferring a relative risk of 3 in the UK.[20]
Having other autoimmune diseases is a risk factor to develop Hashimoto’s thyroiditis, and the opposite is also true.[1] Autoimmune diseases most commonly associated to Hashimoto’s thyroiditis include celiac disease, type 1 diabetes, vitiligo, and alopecia.[21]
Preventable environmental factors, including high iodine intake, selenium deficiency, as well as infectious diseases and certain drugs, have been implicated in the development of autoimmune thyroid disease in genetically predisposed individuals.[22]
The genes implicated vary in different ethnic groups and the incidence is increased in people with chromosomal disorders, including Turner, Down, and Klinefelter syndromes usually associated with autoantibodies against thyroglobulin and thyroperoxidase. Progressive depletion of these cells as the cytotoxic immune response leads to higher degrees of primary hypothyroidism, presenting with low T3/T4 levels, and compensatory elevations of TSH.
Pathophysiology
There are multiple suggested mechanisms by which the pathology of Hashimoto's thyroiditis develops.
Various autoantibodies may be present against thyroid peroxidase, thyroglobulin and TSH receptors, although a small percentage of people may have none of these antibodies present. As indicated in various twin studies, a percentage of the population may also have these antibodies without developing Hashimoto's thyroiditis. Nevertheless, antibody-dependent cell-mediated cytotoxicity is a substantial factor behind the apoptotic fall-out of Hashimoto's thyroiditis. Activation of cytotoxic T-lymphocytes (CD8+ T-cells) in response to cell-mediated immune response affected by helper T-lymphocytes (CD4+ T-cells) is central to thyrocyte destruction. As is characteristic of type IV hypersensitivities, recruitment of macrophages is another effect of the helper T-lymphocyte activation, with Th1 axis lymphocytes producing inflammatory cytokines within thyroid tissue to further macrophage activation and migration into the thyroid gland for direct effect.
Gross morphological changes within the thyroid are seen in the general enlargement, which is far more locally nodular and irregular than more diffuse patterns (such as that of hyperthyroidism). While the capsule is intact and the gland itself is still distinct from surrounding tissue, microscopic examination can provide a more revealing indication of the level of damage.[23]
Histologically, the hypersensitivity is seen as diffuse parenchymal infiltration by lymphocytes, particularly plasma B-cells, which can often be seen as secondary lymphoid follicles (germinal centers, not to be confused with the normally present colloid-filled follicles that constitute the thyroid). Atrophy of the colloid bodies is lined by Hürthle cells, cells with intensely eosinophilic, granular cytoplasm, a metaplasia from the normal cuboidal cells that constitute the lining of the thyroid follicles. Severe thyroid atrophy presents often with denser fibrotic bands of collagen that remains within the confines of the thyroid capsule.[24]
Diagnosis
Diagnosis is usually made by detecting elevated levels of anti-thyroid peroxidase antibodies (TPOAb) in the serum, but seronegative (without circulating autoantibodies) thyroiditis is also possible.[25]
Given the relatively non-specific symptoms of initial hypothyroidism, Hashimoto's thyroiditis is often misdiagnosed as depression, cyclothymia, PMS, chronic fatigue syndrome, fibromyalgia and, less frequently, as erectile dysfunction or an anxiety disorder. On gross examination, there is often presentation of a hard goiter that is not painful to the touch;[23] other symptoms seen with hypothyroidism, such as periorbital myxedema, depend on the current state of progression of the response, especially given the usually gradual development of clinically relevant hypothyroidism. Testing for thyroid-stimulating hormone (TSH), free T3, free T4, and the anti-thyroglobulin antibodies (anti-Tg), anti-thyroid peroxidase antibodies (anti-TPO, or TPOAb) and anti-microsomal antibodies can help obtain an accurate diagnosis.[26] Earlier assessment of the person may present with elevated levels of thyroglobulin owing to transient thyrotoxicosis, as inflammation within the thyroid causes damage to the integrity of thyroid follicle storage of thyroglobulin; TSH secretion from the anterior pituitary increases in response to a decrease in negative feedback inhibition secondary to decreased serum thyroid hormones. Typically T4 is the preferred thyroid hormone test for hypothyroidism.[27] This exposure of the body to substantial amounts of previously isolated thyroid enzymes is thought to contribute to the exacerbation of tolerance breakdown, giving rise to the more pronounced symptoms seen later in the disease. Lymphocytic infiltration of the thyrocyte-associated tissues often leads to the histologically significant finding of germinal center development within the thyroid gland.
Hashimoto's when presenting as mania is known as Prasad's syndrome after Ashok Prasad, the psychiatrist who first described it.[28]
Treatment
Managing hormone levels
Hypothyroidism caused by Hashimoto's thyroiditis is treated with thyroid hormone replacement agents such as levothyroxine, triiodothyronine or desiccated thyroid extract. A tablet taken once a day generally keeps the thyroid hormone levels normal. In most cases, the treatment needs to be taken for the rest of the person's life. In the event that hypothyroidism is caused by Hashimoto's thyroiditis, it may be recommended that the TSH levels be kept under 3.0 mIU/L.[29]
Prognosis
Overt, symptomatic thyroid dysfunction is the most common complication, with about 5% of people with subclinical hypothyroidism and chronic autoimmune thyroiditis progressing to thyroid failure every year. Transient periods of thyrotoxicosis (over-activity of the thyroid) sometimes occur, and rarely the illness may progress to full hyperthyroid Graves' disease with active orbitopathy (bulging, inflamed eyes). Rare cases of fibrous autoimmune thyroiditis present with severe dyspnea (shortness of breath) and dysphagia (difficulty swallowing), resembling aggressive thyroid tumors – but such symptoms always improve with surgery or corticosteroid therapy. Primary thyroid B cell lymphoma affects fewer than one in a thousand persons, and it is more likely to affect those with long-standing autoimmune thyroiditis.[30]
Epidemiology
This disorder is thought to be the most common cause of primary hypothyroidism in North America; as a cause of non-endemic goiter, it is among the most common.[31] Hashimoto's thyroiditis affects about 5% of Caucasians at some point in their life.[4] About 1 to 1.5 in 1000 people have this disease at any point in time.[31] It occurs between eight and fifteen times more often in women than in men. Though it may occur at any age, including in children, it is most often observed in women between 30 and 60 years of age.[30] Some research suggests a connection to the role of the placenta as an explanation for the sex difference.[32]
It is more common in regions of high iodine dietary intake, and among people who are genetically susceptible.[30]
History
Also known as Hashimoto's disease, Hashimoto's thyroiditis is named after the Japanese physician Hakaru Hashimoto (1881−1934) of the medical school at Kyushu University,[33] who first described the symptoms of persons with struma lymphomatosa, an intense infiltration of lymphocytes within the thyroid, in 1912 in a German publication.[34]
In 1957 it was recognized as an autoimmune disorder and was the first organ-specific one identified.[10]
Pregnancy
Pregnant women who are positive for Hashimoto's thyroiditis may have decreased thyroid function or the gland may fail entirely.[35] If a woman is TPOAb-positive, clinicians can inform her of the risks for themselves and their infants if they go untreated. "Thyroid peroxidase antibodies (TPOAb) are detected in 10% of pregnant women," which presents risks to those pregnancies.[35] Women who have low thyroid function that has not been stabilized are at greater risk of having an infant with: low birth weight, neonatal respiratory distress, hydrocephalus, hypospadias, miscarriage, and preterm delivery.[35][36] The embryo transplantion rate and successful pregnancy outcomes are improved when Hashimoto's is treated.[36] Recommendations are to only treat pregnant women who are TPOAb-positive throughout the entirety of their pregnancies and to screen all pregnant women for thyroid levels.[35] Close cooperation between the endocrinologist and obstetrician benefits the woman and the infant.[35][37][38] The Endocrine Society recommends screening in pregnant women who are considered high-risk for thyroid autoimmune disease.[39]
Thyroid peroxides antibodies testing is recommended for women who have ever been pregnant regardless of pregnancy outcome. "...[P]revious pregnancy plays a major role in development of autoimmune overt hypothyroidism in premenopausal women, and the number of previous pregnancies should be taken into account when evaluating the risk of hypothyroidism in a young women [sic]."[40]
See also
- Hashimoto's encephalopathy
- Myxedematous psychosis
References
- "Hashimoto's Disease". NIDDK. May 2014. Archived from the original on 22 August 2016. Retrieved 9 August 2016.
- Noureldine, SI; Tufano, RP (January 2015). "Association of Hashimoto's thyroiditis and thyroid cancer". Current Opinion in Oncology. 27 (1): 21–5. doi:10.1097/cco.0000000000000150. PMID 25390557.
- Hiromatsu, Y; Satoh, H; Amino, N (2013). "Hashimoto's thyroiditis: history and future outlook". Hormones (Athens, Greece). 12 (1): 12–8. doi:10.1007/BF03401282. PMID 23624127.
- Pyzik, A; Grywalska, E; Matyjaszek-Matuszek, B; Roliński, J (2015). "Immune disorders in Hashimoto's thyroiditis: what do we know so far?". Journal of Immunology Research. 2015: 979167. doi:10.1155/2015/979167. PMC 4426893. PMID 26000316.
- Akamizu, T; Amino, N; DeGroot, LJ; De Groot, LJ; Beck-Peccoz, P; Chrousos, G; Dungan, K; Grossman, A; Hershman, JM; Koch, C; McLachlan, R; New, M; Rebar, R; Singer, F; Vinik, A; Weickert, MO (2000). "Hashimoto's Thyroiditis". PMID 25905412. Cite journal requires
|journal=(help) - "Hashimoto's disease". Office on Women’s Health, U.S. Department of Health and Human Services. 12 June 2017. Archived from the original on 28 July 2017. Retrieved 17 July 2017.

- "Hashimoto Thyroiditis – Endocrine and Metabolic Disorders". Merck Manuals Professional Edition. July 2016. Retrieved 30 December 2017.
- "Hashimoto Thyroiditis – Hormonal and Metabolic Disorders". Merck Manuals Consumer Version. Retrieved 30 December 2017.
- Shoenfeld, Yehuda; Cervera, Ricard; Gershwin, M. Eric (2010). Diagnostic Criteria in Autoimmune Diseases. Springer Science & Business Media. p. 216. ISBN 9781603272858.
- Moore, Elaine A.; Wilkinson, Samantha (2009). The Promise of Low Dose Naltrexone Therapy: Potential Benefits in Cancer, Autoimmune, Neurological and Infectious Disorders. McFarland. p. 30. ISBN 9780786452583.
- "Hashimoto's disease – Symptoms and causes". Mayo Clinic. Retrieved 5 October 2018.
- "Hashimoto's disease fact sheet". Office on Women's Health, U.S. Department of Health and Human Services, womenshealth.gov (or girlshealth.gov). 16 July 2012. Archived from the original on 2 December 2014. Retrieved 23 November 2014.
- Page 56 in: Staecker, Hinrich; Thomas R. Van De Water; Van de Water, Thomas R. (2006). Otolaryngology: basic science and clinical review. Stuttgart: Thieme. ISBN 978-0-86577-901-3.
- "Pathogenesis of Hashimoto's thyroiditis (chronic autoimmune thyroiditis)". UpToDate.
- "Hashimoto Thyroiditis". NCBI StatPearls. 2019.
- Dayan CM, Daniels GH (1996). "Chronic autoimmune thyroiditis". American Family Physician. 61 (4): 335:99–107. Archived from the original on 13 April 2014. Retrieved 11 April 2014.
- Chistiakov, Dimitry A (2005). "Immunogenetics of Hashimoto's thyroiditis". Journal of Autoimmune Diseases. 2 (1): 1. doi:10.1186/1740-2557-2-1. PMC 555850. PMID 15762980.
- Kavvoura, F. K.; Akamizu, T.; Awata, T.; Ban, Y.; Chistiakov, D. A.; Frydecka, I.; Ghaderi, A.; Gough, S. C.; Hiromatsu, Y. (2007). "Cytotoxic T-Lymphocyte Associated Antigen 4 Gene Polymorphisms and Autoimmune Thyroid Disease: A Meta-Analysis". Journal of Clinical Endocrinology & Metabolism. 92 (8): 3162–70. doi:10.1210/jc.2007-0147. PMID 17504905.
- Jacobson, Eric M.; Tomer, Yaron (2007). "The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future". Journal of Autoimmunity. 28 (2–3): 85–98. doi:10.1016/j.jaut.2007.02.006. PMC 2043086. PMID 17369021.
- Tandon, N.; Zhang, L.; Weetman, A. P. (May 1991). "HLA associations with Hashimoto's thyroiditis". Clinical Endocrinology. 34 (5): 383–386. doi:10.1111/j.1365-2265.1991.tb00309.x. ISSN 0300-0664. PMID 1676351.
- Radetti G (2014). Clinical aspects of Hashimoto's thyroiditis. Endocr Dev (Review). Endocrine Development. 26. pp. 158–70. doi:10.1159/000363162. ISBN 978-3-318-02720-4. PMID 25231451.
- Saranac, L.; Zivanovic, S.; Bjelakovic, B.; Stamenkovic, H.; Novak, M.; Kamenov, B. (2011). "Why is the Thyroid So Prone to Autoimmune Disease". Hormone Research in Paediatrics. 75 (3): 157–65. doi:10.1159/000324442. PMID 21346360.
- Kumar, Vinay (2010). "24: The Endocrine System". Robbins and Cotran Pathologic Mechanisms of Disease (8th ed.). Philadelphia, PA: Elsevier. p. 1113.
- Kumar, Vinay (2010). "24: The Endocrine System". Robbins and Cotran Pathologic Mechanisms of Disease (8th ed.). Philadelphia, PA: Elsevier. pp. 1112–1113.
- Grani, Giorgio; Carbotta, Giovanni; Nesca, Angela; D’Alessandri, Mimma; Vitale, Martina; Del Sordo, Marianna; Fumarola, Angela (4 October 2014). "A comprehensive score to diagnose Hashimoto's thyroiditis: a proposal". Endocrine. 49 (2): 361–5. doi:10.1007/s12020-014-0441-5. PMID 25280964.
- Giannini, AJ (1986). The Biological Foundations of Clinical Psychiatry. New Hyde Park, NY: Medical Examination Publishing Company. pp. 193–198. ISBN 978-0-87488-449-4.
- "Hashimoto Thyroiditis Workup: Approach Considerations, Serum TSH Test and Other Studies, Ultrasonography". emedicine.medscape.com. Retrieved 4 December 2017.
- "Prasad's syndrome". Archived from the original on 9 June 2011.
- "Does Your Doctor Know About the New TSH Lab Standards?". Archived from the original on 4 December 2010.
- Fabrizio Monaco (2012). Thyroid Diseases. Taylor and Francis. p. 78. ISBN 9781439868393.
- Kumar, Vinay (2010). "24: The Endocrine System". Robbins and Cotran Pathologic Mechanisms of Disease (8th ed.). Philadelphia, PA: Elsevier. pp. 1111–205.
- Wilson, Melissa A.; Trumble, Benjamin C.; Buetow, Kenneth H.; Garcia, Angela R.; Natri, Heini (1 July 2019). "The Pregnancy Pickle: Evolved Immune Compensation Due to Pregnancy Underlies Sex Differences in Human Diseases". Trends in Genetics. 35 (7): 478–488. doi:10.1016/j.tig.2019.04.008. ISSN 0168-9525. PMC 6611699. PMID 31200807. Retrieved 2 July 2019.
- Hakaru Hashimoto at Who Named It?
- Hashimoto, H. (1912). "Zur Kenntnis der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa)". Archiv für Klinische Chirurgie (in German). 97: 219–248.
- Lepoutre, Thibault; Debiève, Frederic; Gruson, Damien; Daumerie, Chantal (1 January 2012). "Reduction of Miscarriages through Universal Screening and Treatment of Thyroid Autoimmune Diseases". Gynecologic and Obstetric Investigation. 74 (4): 265–273. doi:10.1159/000343759. PMID 23147711.
- Zaletel, Katja (2011). "Thyroid physiology and autoimmunity in pregnancy and after delivery". Expert Review of Clinical Immunology. 7 (5): 697–707. doi:10.1586/eci.11.42. PMID 21895480. Retrieved 4 October 2015.
- Budenhofer, Brigitte K.; Ditsch, Nina; Jeschke, Udo; Gärtner, Roland; Toth, Bettina (27 October 2012). "Thyroid (dys-)function in normal and disturbed pregnancy". Archives of Gynecology and Obstetrics. 287 (1): 1–7. doi:10.1007/s00404-012-2592-z. ISSN 0932-0067. PMID 23104052.
- Balucan, Francis S.; Morshed, Syed A.; Davies, Terry F. (1 January 2013). "Thyroid Autoantibodies in Pregnancy: Their Role, Regulation and Clinical Relevance". Journal of Thyroid Research. 2013: 182472. doi:10.1155/2013/182472. PMC 3652173. PMID 23691429.
- "Endocrine Experts Support Screening for Thyroid Dysfunction in Pregnant Women". Endocrine Society. Endocrine Society. 26 March 2015. Archived from the original on 8 October 2015. Retrieved 4 October 2015.
- Carlé, Allan; Pedersen, Inge Bülow; Knudsen, Nils; Perrild, Hans; Ovesen, Lars; Rasmussen, Lone Banke; Laurberg, Peter (2 April 2014). "Development of Autoimmune Overt Hypothyroidism Is Highly Associated With Live Births and Induced Abortions but Only in Premenopausal Women". The Journal of Clinical Endocrinology & Metabolism. 99 (6): 2241–2249. doi:10.1210/jc.2013-4474. ISSN 0021-972X. PMID 24694338.
External links
| Classification | |
|---|---|
| External resources |