Congenital adrenal hyperplasia due to 21-hydroxylase deficiency
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency in all its forms, accounts for over 95% of diagnosed cases of congenital adrenal hyperplasia,[1] and CAH in most contexts refers to 21-hydroxylase deficiency and different mutations related to enzyme impairment have been mapped on protein structure[2].
| Congenital adrenal hyperplasia due to 21-hydroxylase deficiency | |
|---|---|
| Other names | 21-OH CAH |
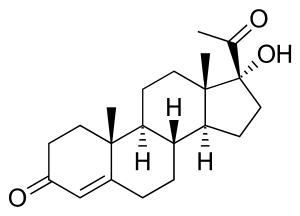 | |
| Deficient 21-Hydroxylase can lead to accumulation of 17α-Hydroxyprogesterone | |
| Specialty | Endocrinology |
Types
Severe, early onset 21-hydroxylase deficient CAH
The two most serious neonatal consequences of 21-hydroxylase deficiency occur when there is minimal measurable hydroxylase activity from prenatal life: life-threatening salt-wasting crises in the first month of life for XX and XY infants alike and severe virilization of female infants.
Salt-wasting crises in infancy
The excessive amounts of adrenal testosterone produce little effect on the genitalia of male infants with severe CAH. If a male infant with CAH is not detected by newborn screening, he will appear healthy and normal and be quickly discharged home to his family.
However, the lack of aldosterone results in a high rate of sodium loss in the urine. Urinary sodium concentrations may exceed 50 mEq/L. With this rate of salt loss, the infant cannot maintain blood volume, and hyponatremic dehydration begins to develop by the end of the first week of life. Potassium and acid excretion are also impaired when mineralocorticoid activity is deficient, and hyperkalemia and metabolic acidosis gradually develop. Ability to maintain circulation is further limited by the effect of cortisol deficiency. The early symptoms are spitting and poor weight gain, but most infants with severe CAH develop vomiting, severe dehydration, and circulatory collapse (shock) by the second or third week of life.
When brought to a hospital, the 1-3 week old infant will be both underweight and dehydrated by appearance. Blood pressure may be low. Basic chemistries will reveal hyponatremia, with a serum Na+ typically between 105 and 125 mEq/L. Hyperkalemia in these infants can be extreme—levels of K+ above 10 mEq/L are not unusual—as can the degree of metabolic acidosis. Hypoglycemia may be present. This is termed a salt-wasting crisis and rapidly causes death if not treated.
As ill as these infants can be, they respond rapidly to treatment with hydrocortisone and intravenous saline and dextrose quickly restores blood volume, blood pressure, and body sodium content, and reverses the hyperkalemia. With appropriate treatment, most infants are out of danger within 24 hours.
Virilization of female infants
Virilization of genetically female (XX) infants usually produces obvious genital ambiguity. Inside the pelvis, the ovaries are normal and since they have not been exposed to testicular antimullerian hormone (AMH), the uterus, fallopian tubes, upper vagina, and other mullerian structures are normally formed as well. However, the high levels of testosterone in the blood can enlarge the phallus, partially or completely close the vaginal opening, enclose the urethral groove so that it opens at the base of the phallus, on the shaft or even at the tip like a boy. Testosterone can cause the labial skin to become as thin and rugated as a scrotum, but cannot produce palpable gonads (i.e., testes) in the folds.
Thus, depending on the severity of hyperandrogenism, a female infant can be mildly affected, obviously ambiguous, or so severely virilized as to appear to be a male. Andrea Prader devised the following Prader scale as a way of describing the degree of virilization.
- An infant at stage 1 has a mildly large clitoris and slightly reduced vaginal opening size. This degree may go unnoticed or may be simply assumed to be within normal variation.
- Stages 2 and 3 represent progressively more severe degrees of virilization. The genitalia are obviously abnormal to the eye, with a phallus intermediate in size and a small vaginal opening.
- Stage 4 looks more male than female, with an empty scrotum and a phallus the size of a normal penis, but not quite free enough of the perineum to be pulled onto the abdomen toward the umbilicus (i.e., what is termed a chordee in a male). The single small urethral/vaginal opening at the base or on the shaft of the phallus would be considered a hypospadias in a male. X-rays taken after dye injection into this opening reveal the internal connection with the upper vagina and uterus. This common opening can predispose to urinary obstruction and infection.
- Stage 5 denotes complete male virilization, with a normally formed penis with the urethral opening at or near the tip. The scrotum is normally formed but empty. The internal pelvic organs include normal ovaries and uterus, and the vagina connects internally with the urethra as in Stage 4. These infants are not visibly ambiguous, and are usually assumed to be ordinary boys with undescended testes. In most cases, the diagnosis of CAH is not suspected until signs of salt-wasting develop a week later.
When the genitalia are determined to be ambiguous at birth, CAH is one of the leading diagnostic possibilities. Evaluation reveals the presence of a uterus, extreme elevation of 17OHP, levels of testosterone approaching or exceeding the male range but low AMH levels. The karyotype is that of an ordinary female: 46,XX. With this information, the diagnosis of CAH is readily made and female sex confirmed.
Evaluation of ambiguous genitalia is described in detail elsewhere. In most cases it is possible to confirm and assign female sex within 12–36 hours of birth. The exception are the rare, completely virilized genetic females (Prader stage 5), who present the most challenging assignment and surgery dilemmas, discussed below.
When the degree of ambiguity is obvious, corrective surgery is usually offered and performed. As reconstructive surgery on infant genitalia has become a focus of controversy, the issues are described in more detail below.
Sex assignment issues and controversies
There are no difficulties assigning appropriate sex for most infants with CAH. Genetic males have normal male genitalia and gonads and simply need hormone replacement. Most virilized females are assigned and raised as girls even if their genitalia are ambiguous or look more male than female. They have normal ovaries and uterus and potential fertility with hormone replacement and surgery. However, the dilemmas surrounding sex assignment of the most severely virilized XX infants have helped shape our understanding of gender identity and sexual orientation, and continue to be a subject of debate.
Until the 1950s, some virilized XX infants were assigned and raised as girls, and some as boys. Most developed gender identities congruent with their sex of rearing. In a few cases of male rearing, a sex reassignment was attempted in mid-childhood when newly discovered karyotyping revealed "female" chromosomes. These reassignments were rarely successful, leading John Money and other influential psychologists and physicians to conclude that gender identity was (1) unrelated to chromosomes, (2) primarily a result of social learning, and (3) could not be easily changed after infancy.
By the 1960s, CAH was well understood, karyotyping was routine, and standard management was to assign and raise all children with CAH according to their gonads and karyotypes, no matter how virilized. Markedly virilized girls were usually referred to a pediatric surgeon, often a pediatric urologist for a reconstructive vaginoplasty and clitoral reduction or recession—surgery to create or enlarge a vaginal opening and reduce the size or protrusion of the clitoris. This approach was designed to preserve fertility for both sexes and remains the standard management, but two aspects of this management have been challenged: assignment of completely virilized genetic females and the value and age of corrective surgery.
The first questions about assignment were raised in the early 1980s when Money and others reported an unexpectedly high rate of failure to achieve normal adult sexual relationships (i.e., heterosexual orientation, marriage, and children) in grown women with CAH (though all had female gender identities). However, the sample was small, and results seemed interpretable in many ways: selection bias, early hormone effects on orientation, or sexual dysfunction created by residual body abnormalities or by the genital surgery itself. From a perspective two decades later, the report was one of the first pieces of evidence that the standard management paradigm was not always producing hoped-for outcomes.
Despite these concerns, no significant opposition to standard management arose until the mid-1990s, when a confluence of evidence and opinion from several sources led to a re-examination of outcomes. Several intersex support and advocacy groups (e.g., the Intersex Society of North America) began to publicly criticize infant genital surgery based on unsatisfactory outcomes of some adults who had been operated on as infants. Their complaints were that they had reduced ability to enjoy sexual relations or that they resented not having had the choice of gender assignment or surgical reconstruction left until they were old enough to participate. (See History of intersex surgery.)
In 1997, influential articles by Reiner, Diamond, and Sigmundson advocated consideration of (1) male sex assignment in the unambiguously male XX infants (most of whom are considered male until the CAH is recognized at 1–2 weeks of age), and (2) delaying reconstructive surgery until the patient is old enough to participate in the decision. (See Ambiguous genitalia and Intersex for more on this debate, as well as complete citations.)
Although the standard management approach remains "standard", more time and consideration are being given in many cases to explaining alternatives to parents and a small number of XX children with unambiguously male external genitalia are again being raised as boys.
Childhood-onset (simple virilizing) CAH
Mutations that result in some residual 21-hydroxylase activity cause milder disease, traditionally termed simple virilizing CAH (SVCAH). In these children the mineralocorticoid deficiency is less significant and salt-wasting does not occur. However, genital ambiguities are possible.
Late onset (nonclassical) CAH
The androgen excess is mild enough that virilization is not apparent or goes unrecognized at birth and in early childhood. However, androgen levels are above normal and slowly rise during childhood, producing noticeable effects between 2 and 9 years of age.
Appearance of pubic hair in mid-childhood is the most common feature that leads to evaluation and diagnosis. Other accompanying features are likely to be tall stature and accelerated bone age (often 3–5 years ahead). Often present are increased muscle mass, acne, and adult body odor. In boys the penis will be enlarged. Mild clitoral enlargement may occur in girls, and sometimes a degree of prenatal virilization is recognized that may have gone unnoticed in infancy.
The principal goals of treatment of non-classical CAH are to preserve as much growth as possible and to prevent central precocious puberty if it has not already been triggered. These are more difficult challenges than in CAH detected in infancy because moderate levels of androgens will have had several years to advance bone maturation and to trigger central puberty before the disease is detected.
A diagnosis of non-classical CAH is usually confirmed by discovering extreme elevations of 17α-hydroxyprogesterone along with moderately high testosterone levels. A cosyntropin stimulation test may be needed in mild cases, but usually the random levels of 17OHP are high enough to confirm the diagnosis.
The mainstay of treatment is suppression of adrenal testosterone production by a glucocorticoid such as hydrocortisone. Mineralocorticoid is only added in cases where the plasma renin activity is high.
A third key aspect of management is suppression of central precocious puberty if it has begun. The usual clues to central puberty in boys are that the testes are pubertal in size, or that testosterone remains elevated even when the 17OHP has been reduced toward normal. In girls central puberty is less often a problem, but breast development would be the main clue. Central precocious puberty is suppressed when appropriate by leuprolide.
As outlined above, recent additions to treatment to preserve growth include aromatase inhibition to slow bone maturation by reducing the amount of testosterone converted to estradiol, and use of blockers of estrogen for the same purpose.
Once adrenal suppression has been achieved, the patient needs stress steroid coverage as described above for significant illness or injury.
Other alleles result in even milder degrees of hyperandrogenism that may not even cause problems in males and may not be recognized until adolescence or later in females. Mild androgen effects in young women may include hirsutism, acne, or anovulation (which in turn can cause infertility). Testosterone levels in these women may be mildly elevated, or simply above average. These clinical features are those of polycystic ovary syndrome (PCOS), and a small percentage of women with PCOS are found to have late-onset CAH when investigated.
Diagnosis of late-onset CAH may be suspected from a high 17α-hydroxyprogesterone level, but some cases are so mild that the elevation is only demonstrable after cosyntropin stimulation. Treatment may involve a combination of very low dose glucocorticoid to reduce adrenal androgen production and any of various agents to block the androgen effects and/or induce ovulation.
It was characterized in 1979 by Dr. Maria New.[3] Prevalence has been described as 1 in 100 in certain populations.[4]
Presentation
Testicular adrenal rest tumors
Infertility observed in adult males with congenital adrenal hyperplasia (CAH) has been associated with testicular adrenal rest tumors (TART) that may originate during childhood. TART in prepubertal males with classic CAH could be found during childhood (20%). Martinez-Aguayo et al. reported differences in markers of gonadal function in a subgroup of patients, especially in those with inadequate control.[5]
Genetics
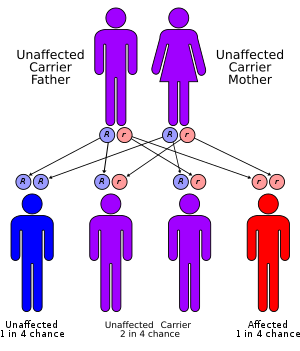
The CYP21A2 gene for the P450c21 enzyme (also known as 21-hydroxylase) is at 6p21.3,[6] amid genes HLA B and HLA DR coding for the major human histocompatibility loci (HLA). CYP21A2 is paired with a nonfunctional pseudogene CYP21A1P. Scores of abnormal alleles of CYP21A2 have been documented, most arising from recombinations of homologous regions of CYP21A2 and CYP21A1P. Differences in residual enzyme activity of the various alleles account for the various degrees of severity of the disease. Inheritance of all forms of 21-hydroxylase CAH is autosomal recessive.
Persons affected by any forms of the disease have two abnormal alleles, and both parents are usually heterozygotes (or carriers). When both parents carry an abnormal allele, each child has a 25% chance of having the disease, a 50% chance of being a carrier like the parents, and a 25% chance of having two normal genes.
It is now possible to test for heterozygosity by measuring 17α-hydroxyprogesterone elevation after ACTH stimulation, or more recently by direct gene sequencing.
Pathophysiology
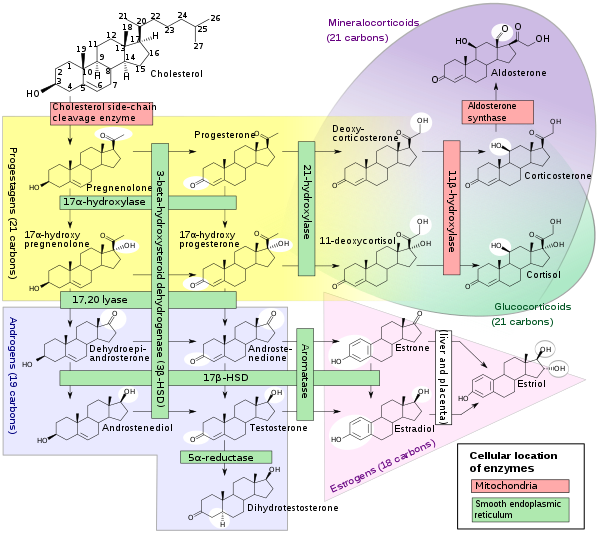
The enzyme P450c21, commonly referred to as 21-hydroxylase (21-OH), is embedded in the smooth endoplasmic reticulum of the cells of the adrenal cortex. It catalyzes hydroxylation of 17α-hydroxyprogesterone (17OHP) to 11-deoxycortisol in the glucocorticoid pathway, which starts from pregnenolone and finishes with cortisol. It also catalyzes hydroxylation of progesterone to 11-deoxycorticosterone (DOC) in the mineralocorticoid pathway on its way from pregnenolone to aldosterone.
Deficient activity of this enzyme reduces the efficiency of cortisol synthesis, with consequent hyperplasia of the adrenal cortex and elevation of ACTH levels. ACTH stimulates uptake of cholesterol and synthesis of pregnenolone. Steroid precursors up to and including progesterone, 17α-hydroxypregnenolone, and especially 17α-hydroxyprogesterone accumulate in the adrenal cortex and in circulating blood. Blood levels of 17OHP can reach 10-1000 times the normal concentration.
Since 21-hydroxylase activity is not involved in synthesis of androgens, a substantial fraction of the large amounts of 17α-hydroxypregnenolone is diverted to synthesis of DHEA, androstenedione, and testosterone beginning in the third month of fetal life in both sexes.
Synthesis of aldosterone is also dependent on 21-hydroxylase activity. Although fetal production is impaired, it causes no prenatal effects, as the placental connection allows maternal blood to "dialyze" the fetus and maintain both electrolyte balance and blood volume.
Diagnosis
Since CAH is an autosomal recessive disease, most children with CAH are born to parents unaware of the risk and with no family history. Each child will have a 25% chance of being born with the disease. Families typically wish to minimize the degree of virilization of a girl. There is no known prenatal harm to a male fetus from CAH, so treatment can begin at birth.
Adrenal glands of female fetuses with CAH begin producing excess testosterone by the 9th week of gestation. The most important aspects of virilization (urogenital closure and phallic urethra) occur between 8 and 12 weeks. Theoretically, if enough glucocorticoid could be supplied to the fetus to reduce adrenal testosterone production by the 9th week, virilization could be prevented and the difficult decision about timing of surgery avoided.
The challenge of preventing severe virilization of girls is twofold: detection of CAH at the beginning of the pregnancy, and delivery of an effective amount of glucocorticoid to the fetus without causing harm to the mother.
Classification
The condition can be classified into "salt-wasting", "simple virilizing", and "non-classical" forms.
| Type | Sex steroid effects | Other effects |
|---|---|---|
| Severe 21-hydroxylase deficiency causes salt-wasting CAH | The most common cause of ambiguous genitalia due to prenatal virilization of genetically female (XX) infants. | Life-threatening vomiting and dehydration occurring within the first few weeks of life. Aldosterone and cortisol levels are both reduced. |
| Moderate 21-hydroxylase deficiency is referred to as simple virilizing CAH | Typically is recognized as causing virilization of prepubertal children. | Cortisol is reduced, but aldosterone is not. |
| Still milder forms of 21-hydroxylase deficiency are referred to as non-classical CAH | Can cause androgen effects and infertility in adolescent and adult women. | Neither aldosterone nor cortisol are reduced. |
The salt-wasting and simple virilizing types are sometimes grouped together as "classical".[7]
Screening
Conditions justifying newborn screening for any disorder include (1) a simple test with an acceptable sensitivity and specificity, (2) a dire consequence if not diagnosed early, (3) an effective treatment if diagnosed, and (4) a frequency in the population high enough to justify the expense. In the last decade more states and countries are adopting newborn screening for salt-wasting CAH due to 21-hydroxylase deficiency, which leads to death in the first month of life if not recognized.
The salt-wasting form of CAH has an incidence of 1 in 15,000 births and is potentially fatal within a month if untreated. Steroid replacement is a simple, effective treatment. However, the screening test itself is less than perfect. While the 17α-hydroxyprogesterone level is easy to measure and sensitive (rarely missing real cases), the test has a poorer specificity. Screening programs in the United States have reported that 99% of positive screens turn out to be false positives upon investigation of the infant. This is a higher rate of false positives than the screening tests for many other congenital metabolic diseases.
When a positive result is detected, the infant must be referred to a pediatric endocrinologist to confirm or disprove the diagnosis. Since most infants with salt-wasting CAH become critically ill by 2 weeks of age, the evaluation must be done rapidly despite the high false positive rate.
Levels of 17α-hydroxyprogesterone, androstenedione, and cortisol may play a role in screening.[8]
Treatment
The first problem has not yet been entirely solved, but it has been shown that if dexamethasone is taken by a pregnant woman, enough can cross the placenta to suppress fetal adrenal function.
At present no program screens for risk in families who have not yet had a child with CAH. For families desiring to avoid virilization of a second child, the current strategy is to start dexamethasone as soon as a pregnancy has been confirmed even though at that point the chance that the pregnancy is a girl with CAH is only 12.5%. Dexamethasone is taken by the mother each day until it can be safely determined whether she is carrying an affected girl.
Whether the fetus is an affected girl can be determined by chorionic villus sampling at 9–11 weeks of gestation, or by amniocentesis at 15–18 weeks gestation. In each case the fetal sex can be determined quickly, and if the fetus is a male the dexamethasone can be discontinued. If female, fetal DNA is analyzed to see if she carries one of the known abnormal alleles of the CYP21 gene. If so, dexamethasone is continued for the remainder of the pregnancy at a dose of about 1 mg daily.
Most mothers who have followed this treatment plan have experienced at least mild cushingoid effects from the glucocorticoid but have borne daughters whose genitalia are much less virilized.
Long-term management of CAH
Management of infants and children with CAH is complex and warrants long term care in a pediatric endocrine clinic. After the diagnosis is confirmed, and any salt-wasting crisis averted or reversed, major management issues include:
- Initiating and monitoring hormone replacement
- Stress coverage, crisis prevention, parental education
- Reconstructive surgery
- Optimizing growth
- Optimizing androgen suppression and fertility in women with CAH
Hormone replacement
The primary goals of hormone replacement are to protect from adrenal insufficiency and to suppress the excessive adrenal androgen production.
Glucocorticoids are provided to all children and adults with all but the mildest and latest-onset forms of CAH. The glucocorticoids provide a reliable substitute for cortisol, thereby reducing ACTH levels. Reducing ACTH also reduces the stimulus for continued hyperplasia and overproduction of androgens. In other words, glucocorticoid replacement is the primary method of reducing the excessive adrenal androgen production in both sexes. A number of glucocorticoids are available for therapeutic use. Hydrocortisone or liquid prednisolone is preferred in infancy and childhood, and prednisone or dexamethasone are often more convenient for adults.
The glucocorticoid dose is typically started at the low end of physiologic replacement (6–12 mg/m2) but is adjusted throughout childhood to prevent both growth suppression from too much glucocorticoid and androgen escape from too little. Serum levels of 17α-hydroxyprogesterone, testosterone, androstenedione, and other adrenal steroids are followed for additional information, but may not be entirely normalized even with optimal treatment. (See Glucocorticoid for more on this topic.)
Mineralocorticoids are replaced in all infants with salt-wasting and in most patients with elevated renin levels. Fludrocortisone is the only pharmaceutically available mineralocorticoid and is usually used in doses of 0.05 to 2 mg daily. Electrolytes, renin, and blood pressure levels are followed to optimize the dose.
Stress coverage, crisis prevention, parental education
Even after diagnosis and initiation of treatment, a small percentage of children and adults with infancy or childhood onset CAH die of adrenal crisis. Deaths from this are entirely avoidable if the child and family understand that the daily glucocorticoids cannot be allowed to be interrupted by an illness. When a person is well, missing a dose, or even several doses, may produce little in the way of immediate symptoms. However, glucocorticoid needs are increased during illness and stress, and missed doses during an illness such as the "flu" (or viral gastroenteritis) can lead within hours to reduced blood pressure, shock, and death.
To prevent this, all persons taking replacement glucocorticoids are taught to increase their doses in the event of illness, surgery, severe injury, or severe exhaustion. More importantly, they are taught that vomiting warrants an injection within hours of hydrocortisone (e.g., SoluCortef) or other glucocorticoid. This recommendation applies to both children and adults. Because young children are more susceptible to vomiting illnesses than adults, pediatric endocrinologists usually teach parents how to give hydrocortisone injections.
As an additional precaution, persons with adrenal insufficiency are advised to wear a medical identification tag or carry a wallet card to alert those who may be providing emergency medical care of the urgent need for glucocorticoids.
Reconstructive surgery
Surgery need never be considered for genetically male (XY) infants because the excess androgens do not produce anatomic abnormality. However, surgery for severely virilized XX infants is often performed and has become a subject of debate in the last decade.
Surgical reconstruction of abnormal genitalia has been offered to parents of severely virilized girls with CAH since the first half of the 20th century. The purposes of surgery have generally been a combination of the following:
- To make the external genitalia look more female than male
- To make it possible for these girls to participate in normal sexual intercourse when they grow up
- To improve their chances of fertility
- To reduce the frequency of urinary infections
In the 1950s and 1960s, surgery often involved clitorectomy (removal of most of the clitoris), an operation that also reduced genital sensation. In the 1970s, new operative methods were developed to preserve innervation and clitoral function. However, a number of retrospective surveys in the last decade suggest that (1) sexual enjoyment is reduced in many women even after nerve-sparing procedures, and (2) women with CAH who have not had surgery also have a substantial rate of sexual dysfunction. (See Intersex surgery for an overview of procedures and potential complications, and History of intersex surgery for a fuller discussion of the controversies.) Many patient advocates and surgeons argue for deferring surgery until adolescence or later, while some surgeons continue to argue that infant surgery has advantages.
Optimizing growth in CAH
One of the challenging aspects of long-term management is optimizing growth so that a child with CAH achieves his or her height potential because both undertreatment and overtreatment can reduce growth or the remaining time for growth. While glucocorticoids are essential for health, dosing is always a matter of approximation. In even mildly excessive amounts, glucocorticoids slow growth. On the other hand, adrenal androgens are readily converted to estradiol, which accelerates bone maturation and can lead to early epiphyseal closure. This narrow target of optimal dose is made more difficult to obtain by the imperfect replication of normal diurnal plasma cortisol levels produced by 2 or 3 oral doses of hydrocortisone. As a consequence, average height losses of about 4 inches (10 cm) have been reported with traditional management.
Traditionally, pediatric endocrinologists have tried to optimize growth by measuring a child every few months to assess current rate of growth, by checking the bone age every year or two, by periodically measuring 17OHP and testosterone levels as indicators of adrenal suppression, and by using hydrocortisone for glucocorticoid replacement rather than longer-acting prednisone or dexamethasone.
The growth problem is even worse in the simple virilizing forms of CAH which are detected when premature pubic hair appears in childhood, because the bone age is often several years advanced at the age of diagnosis. While a boy (or girl) with simple virilizing CAH is taller than peers at that point, he will have far fewer years remaining to grow, and may go from being a very tall 7-year-old to a 62-inch 13-year-old who has completed growth. Even with adrenal suppression, many of these children will have already had central precocious puberty triggered by the prolonged exposure of the hypothalamus to the adrenal androgens and estrogens. If this has begun, it may be advantageous to suppress puberty with a gonadotropin-releasing hormone agonist such as leuprolide to slow continuing bone maturation.
In recent years some newer approaches to optimizing growth have been researched and are beginning to be used. It is possible to reduce the effects of androgens on the body by blocking the receptors with an antiandrogen such as flutamide and by reducing the conversion of testosterone to estradiol. This conversion is mediated by aromatase and can be inhibited by aromatase blockers such as testolactone. Blocking the effects and conversions of estrogens will allow use of lower doses of glucocorticoids with less risk of acceleration of bone maturation. Other proposed interventions have included bilateral adrenalectomy to remove the androgen sources, or growth hormone treatment to enhance growth.
For a more extensive review of the difficulties of optimizing growth, see Migeon CJ, Wisneiewski AB. Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency: growth, development, and therapeutic considerations. Endocrinol Metab Clin N Am 30:193-206, 2001.[9]
Preventing hyperandrogenism and optimizing fertility
As growth ends, management in girls with CAH changes focus to optimizing reproductive function. Both excessive testosterone from the adrenals and excessive glucocorticoid treatment can disrupt ovulation, resulting in irregularity of menses or amenorrhea, as well as infertility. Continued monitoring of hormone balance and careful readjustment of glucocorticoid dose can usually restore fertility, but as a group, women with CAH have a lower fertility rate than a comparable population.
CAH has little effect on male fertility unless an adult stops taking his glucocorticoid medication entirely for an extended time, in which case excessive adrenal testosterone may reduce testicular production as well as spermatogenesis.
Psychosexual development and issues
Nearly all mammals display sex-dimorphic reproductive and sexual behavior (e.g., lordosis and mounting in rodents). Much research has made it clear that prenatal and early postnatal androgens play a role in the differentiation of most mammalian brains. Experimental manipulation of androgen levels in utero or shortly after birth can alter adult reproductive behavior.
Girls and women with CAH constitute the majority of genetic females with normal internal reproductive hormones who have been exposed to male levels of testosterone throughout their prenatal lives. Milder degrees of continuing androgen exposure continue throughout childhood and adolescence as a consequence of the imperfections of current glucocorticoid treatment for CAH. The psychosexual development of these girls and women has been analyzed as evidence of the role of androgens in human sex-dimorphic behaviors.
Girls with CAH have repeatedly been reported to spend more time with "sex-atypical" toys and "rough-and-tumble" play than unaffected sisters. These differences continue into adolescence, as expressed in social behaviors, leisure activities, and career interests. Interest in babies and becoming mothers is significantly lower by most measures.
Cognitive effects are less clear, and reports have been contradictory. Two studies reported spatial abilities above the average for sisters and for girls in general. Other evidence in males with and without androgen deficiencies suggests that androgens may play a role in these aptitudes.
However, gender identity of girls and women with CAH is most frequently observed to be female. Sexual orientation is more mixed, though the majority are heterosexual. In one study, 27% of women with CAH were rated as bisexual in their orientations.
Incidence
The incidence of 21-hydroxylase deficient CAH detectable in childhood is about 1 in 15,000 births. The severe salt-wasting form accounts for the majority of these cases, which is high enough that many states and countries routinely include it in mandated newborn screening tests. The incidence of simple virilizing CAH is about 1 in 60,000 children.
See also
- Inborn errors of steroid metabolism
- Congenital adrenal hyperplasia
- Adrenal insufficiency
- Disorders of sexual development
- Intersexuality, pseudohermaphroditism, and ambiguous genitalia
- 21-Hydroxylase
References
- White PC, Speiser PW (June 2000). "Congenital adrenal hyperplasia due to 21-hydroxylase deficiency". Endocr. Rev. 21 (3): 245–91. doi:10.1210/er.21.3.245. PMID 10857554.
- Neves Cruz, Jorddy; da Costa, Kauê Santana; de Carvalho, Tarcísio André Amorim; de Alencar, Nelson Alberto Nascimento (2020-03-23). "Measuring the structural impact of mutations on cytochrome P450 21A2, the major steroid 21-hydroxylase related to congenital adrenal hyperplasia". Journal of Biomolecular Structure and Dynamics. 38 (5): 1425–1434. doi:10.1080/07391102.2019.1607560. ISSN 0739-1102.
- New MI (December 2004). "An update of congenital adrenal hyperplasia". Ann. N. Y. Acad. Sci. 1038: 14–43. Bibcode:2004NYASA1038...14N. doi:10.1196/annals.1315.009. PMID 15838095.
- New MI (November 2006). "Extensive clinical experience: nonclassical 21-hydroxylase deficiency". J. Clin. Endocrinol. Metab. 91 (11): 4205–14. doi:10.1210/jc.2006-1645. PMID 16912124.
- Martinez-Aguayo, A; Rocha, A; Rojas, N; García, C; Parra, R; Lagos, M; Valdivia, L; Poggi, H; et al. (2007). "Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia". The Journal of Clinical Endocrinology and Metabolism. 92 (12): 4583–9. doi:10.1210/jc.2007-0383. PMID 17895312.
- Trakakis E, Loghis C, Kassanos D (March 2009). "Congenital adrenal hyperplasia because of 21-hydroxylase deficiency. A genetic disorder of interest to obstetricians and gynecologists". Obstet Gynecol Surv. 64 (3): 177–89. doi:10.1097/OGX.0b013e318193301b. PMID 19228439.
- Forest MG, Tardy V, Nicolino M, David M, Morel Y (June 2005). "21-Hydroxylase deficiency: an exemplary model of the contribution of molecular biology in the understanding and management of the disease". Ann. Endocrinol. 66 (3): 225–32. doi:10.1016/s0003-4266(05)81754-8. PMID 15988383.
- Schwarz E, Liu A, Randall H, et al. (April 2009). "Use of Steroid Profiling by UPLC-MS/MS as a Second Tier Test in Newborn Screening for Congenital Adrenal Hyperplasia: the Utah experience". Pediatr. Res. 66 (2): 230–5. doi:10.1203/PDR.0b013e3181aa3777. PMID 19390483.
- Migeon CJ, Wisniewski AB (March 2001). "Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Growth, development, and therapeutic considerations". Endocrinol. Metab. Clin. North Am. 30 (1): 193–206. doi:10.1016/S0889-8529(08)70026-4. PMID 11344936.
External links
| Classification |
|---|
- GeneReviews/NCBI/NIH/UW entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia
- OMIM entry on 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia