Carbonyl fluoride
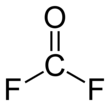
Carbonyl fluoride is a chemical compound with the formula COF2. This gas, like its analog phosgene, is colourless and highly toxic. The molecule is planar with C2v symmetry.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Carbonyl difluoride | |||
| Other names
Fluorophosgene; Carbon difluoride oxide; Fluoromethanoyl fluoride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.005.941 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2417 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| COF2 | |||
| Molar mass | 66.01 g mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 2.698 g/L (gas), 1.139 g/cm3 (liquid at melting point) | ||
| Melting point | −111.26 °C (−168.27 °F; 161.89 K) | ||
| Boiling point | −84.57 °C (−120.23 °F; 188.58 K) | ||
| reacts violently with water[1] | |||
| Vapor pressure | 55.4 atm (20°C)[1] | ||
| Structure | |||
| C2v | |||
| 0.95 D | |||
| Hazards | |||
| Main hazards | Fatal if inhaled, reacts with water | ||
| GHS pictograms |     | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H280, H290, H314, H330, H331, H370 | ||
| P234, P260, P261, P264, P270, P271, P280, P284, P301+330+331, P303+361+353, P304+340, P305+351+338, P307+311, P310, P311, P320, P321, P363, P390, P403+233, P404, P405, P410+403, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[1] | ||
REL (Recommended) |
TWA 2 ppm (5 mg/m3) ST 5 ppm (15 mg/m3)[1] | ||
IDLH (Immediate danger) |
N.D.[1] | ||
| Related compounds | |||
Related compounds |
Phosgene Carbonyl bromide Formyl fluoride Thiocarbonyl chloride Acetone Urea Carbonic acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and properties
Carbonyl fluoride is usually produced as a decomposition product of fluorinated hydrocarbons in the thermal decomposition thereof, for example from trifluoromethanol or tetrafluoromethane in the presence of water:
- CF
4 + H
2O → COF
2 + 2HF
Carbonyl fluoride can also be prepared by reaction of phosgene with hydrogen fluoride and the oxidation of carbon monoxide, although the latter tends to result in over-oxidation to carbon tetrafluoride. The oxidation of carbon monoxide with silver difluoride is convenient:
- CO + 2AgF
2 → COF
2 + 2AgF
Carbonyl fluoride is unstable in the presence of water, hydrolyzing to carbon dioxide and hydrogen fluoride:[2]
- COF
2 + H
2O → CO
2 + 2HF
Safety
Carbonyl fluoride is toxic with a recommended exposure limit of 2 ppm as an 8-hour time weighted average and a 5 ppm as a short-term (15-minute average) exposure.[3]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0108". National Institute for Occupational Safety and Health (NIOSH).
- M. W. Farlow; E. H. Man; C. W. Tullock (1960). "Carbonyl Fluoride". Inorganic Syntheses. 6: 155–158. doi:10.1002/9780470132371.ch48.
- "Carbonyl Fluoride". NIOSH Pocket Guide to Chemical Hazards. CDC Centers for Disease Control and Prevention. Retrieved 2013-09-10.