Adipocyte
Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat.[1] Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. In cell culture, adipocytes can also form osteoblasts, myocytes and other cell types.
| Adipocyte | |
|---|---|
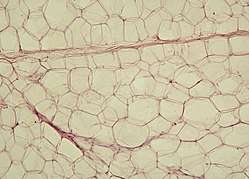 Yellow adipose tissue in paraffin | |
| Details | |
| Identifiers | |
| Latin | adipocytus |
| MeSH | D017667 |
| TH | H2.00.03.0.01005 |
| FMA | 63880 |
| Anatomical terms of microanatomy | |
There are two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), which are also known as white and brown fat, respectively, and comprise two types of fat cells.
Structure
White fat cells (unilocular cells) fasting state
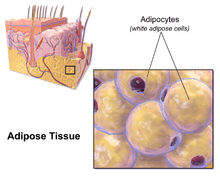
White fat cells or monovacuolar cells contain a large lipid droplet surrounded by a layer of cytoplasm. The nucleus is flattened and located on the periphery. A typical fat cell is 0.1 mm in diameter with some being twice that size and others half that size. The fat stored is in a semi-liquid state, and is composed primarily of triglycerides and cholesteryl ester. White fat cells secrete many proteins acting as adipokines such as resistin, adiponectin, leptin and apelin. An average human adult has 30 billion fat cells with a weight of 30 lbs or 13.5 kg. If excess weight is gained as an adult, fat cells increase in size about fourfold before dividing and increasing the absolute number of fat cells present.[2]
Brown fat cells (multilocular cells)
Brown fat cells or plurivacuolar cells are polyhedral in shape. Unlike white fat cells, these cells have considerable cytoplasm, with lipid droplets scattered throughout. The nucleus is round and, although eccentrically located, it is not in the periphery of the cell. The brown color comes from the large quantity of mitochondria. Brown fat, also known as "baby fat," is used to generate heat.
Marrow fat cells (unilocular cells)
Marrow adipocytes, like brown and white adipocytes, are derived from mesenchymal stem cells. The marrow adipose tissue depot is poorly understood in terms of its physiologic function and relevance to bone health. Marrow adipose tissue expands in states of low bone density but additionally expands in the setting of obesity.[3] Marrow adipose tissue response to exercise approximates that of WAT[3][4][5][6]. Exercise reduces both adipocyte size as well as MAT volume, as quantified by MRI or μCT imaging of bone stained with the lipid binder osmium.
Development
Pre-adipocytes are undifferentiated fibroblasts that can be stimulated to form adipocytes. Recent studies shed light into potential molecular mechanisms in the fate determination of pre-adipocytes although the exact lineage of adipocyte is still unclear.[7][8] The variation of body fat distribution resulting from normal growth is influenced by nutritional and hormonal status in dependence on intrinsic differences in cells found in each adipose depot.[9]
Mesenchymal stem cells can differentiate into adipocytes, connective tissue, muscle or bone.[1]
The term "lipoblast" is used to describe the precursor of the adult cell. The term "lipoblastoma" is used to describe a tumor of this cell type.[10]
Function
Cell turnover
Fat cells in some mice have been shown to drop in count due to fasting and other properties were observed when exposed to cold.[11]
If the adipocytes in the body reach their maximum capacity of fat, they may replicate to allow additional fat storage.
Adult rats of various strains became obese when they were fed a highly palatable diet for several months. Analysis of their adipose tissue morphology revealed increases in both adipocyte size and number in most depots. Reintroduction of an ordinary chow diet[12] to such animals precipitated a period of weight loss during which only mean adipocyte size returned to normal. Adipocyte number remained at the elevated level achieved during the period of weight gain.[13]
In some reports and textbooks, the number of adipocytes can increase in childhood and adolescence, though the amount is usually constant in adults. Individuals who become obese as adults, rather than as adolescents, have no more adipocytes than they had before.[14]
People who have been fat since childhood generally have an inflated number of fat cells. People who become fat as adults may have no more fat cells than their lean peers, but their fat cells are larger. In general, people with an excess of fat cells find it harder to lose weight and keep it off than the obese who simply have enlarged fat cells.[2]
Body fat cells have regional responses to the overfeeding that was studied in adult subjects. In the upper body, an increase of adipocyte size correlated with upper-body fat gain; however, the number of fat cells was not significantly changed. In contrast to the upper body fat cell response, the number of lower-body adipocytes did significantly increase during the course of experiment. Notably, there was no change in the size of the lower-body adipocytes.[15]
Approximately 10% of fat cells are renewed annually at all adult ages and levels of body mass index without a significant increase in the overall number of adipocytes in adulthood.[14]
Adaptation
Obesity is characterized by the expansion of fat mass, through adipocyte size increase (hypertrophy) and, to a lesser extent, cell proliferation (hyperplasia).[16] In the fat cells of obese individuals, there is increased production of metabolism modulators, such as glycerol, hormones, macrophage stimulating chemokines, and pro-inflammatory cytokines, leading to the development of insulin resistance.[17]
Fat production in adipocytes is strongly stimulated by insulin. By controlling the activity of the pyruvate dehydrogenase and the acetyl-CoA carboxylase enzymes, insulin promotes unsaturated fatty acid synthesis. It also promotes glucose uptake and induces SREBF1, which activates the transcription of genes that stimulate lipogenesis.[18]
SREBF1 (sterol regulatory element-binding transcription factor 1) is a transcription factor synthesized as an inactive precursor protein inserted into the endoplasmic reticulum (ER) membrane by two membrane-spanning helices. Also anchored in the ER membrane is SCAP (SREBF-cleavage activating protein), which binds SREBF1. The SREBF1-SCAP complex is retained in the ER membrane by INSIG1 (insulin-induced gene 1 protein). When sterol levels are depleted, INSIG1 releases SCAP and the SREBF1-SCAP complex can be sorted into COPII-coated transport vesicles that are exported to the Golgi. In the Golgi, SREBF1 is cleaved and released as a transcriptionally active mature protein. It is then free to translocate to the nucleus and activate the expression of its target genes.
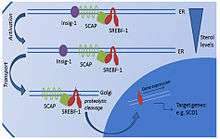
Clinical studies have repeatedly shown that even though insulin resistance is usually associated with obesity, the membrane phospholipids of the adipocytes of obese patients generally still show an increased degree of fatty acid unsaturation.[20] This seems to point to an adaptive mechanism that allows the adipocyte to maintain its functionality, despite the increased storage demands associated with obesity and insulin resistance.
A study conducted in 2013[20] found that, while INSIG1 and SREBF1 mRNA expression was decreased in the adipose tissue of obese mice and humans, the amount of active SREBF1 was increased in comparison with normal mice and non-obese patients. This downregulation of INSIG1 expression combined with the increase of mature SREBF1 was also correlated with the maintenance of SREBF1-target gene expression. Hence, it appears that, by downregulating INSIG1, there is a resetting of the INSIG1/SREBF1 loop, allowing for the maintenance of active SREBF1 levels. This seems to help compensate for the anti-lipogenic effects of insulin resistance and thus preserve adipocyte fat storage abilities and availability of appropriate levels of fatty acid unsaturation in face of the nutritional pressures of obesity.
Endocrine role
Adipocytes can synthesize estrogens from androgens,[21] potentially being the reason why being underweight or overweight are risk factors for infertility.[22] Additionally, adipocytes are responsible for the production of the hormone leptin. Leptin is important in regulation of appetite and acts as a satiety factor.[23]
References
- Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (August 2013). "Role of pericytes in skeletal muscle regeneration and fat accumulation". Stem Cells and Development. 22 (16): 2298–314. doi:10.1089/scd.2012.0647. PMC 3730538. PMID 23517218.
- Robert P (2001). Fat: fighting the obesity epidemic. Oxford [Oxfordshire]: Oxford University Press. pp. 68. ISBN 978-0-19-511853-7.
- Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, Xie Z, Zong X, Styner MA, Rubin CT, Rubin J (August 2017). "Exercise Decreases Marrow Adipose Tissue Through ß-Oxidation in Obese Running Mice". Journal of Bone and Mineral Research. 32 (8): 1692–1702. doi:10.1002/jbmr.3159. PMC 5550355. PMID 28436105.
- Pagnotti GM, Styner M (2016). "Exercise Regulation of Marrow Adipose Tissue". Frontiers in Endocrinology. 7: 94. doi:10.3389/fendo.2016.00094. PMC 4943947. PMID 27471493.
- Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, Sen B, Xie Z, Horowitz MC, Styner MA, Rubin C, Rubin J (August 2015). "Exercise Regulation of Marrow Fat in the Setting of PPARγ Agonist Treatment in Female C57BL/6 Mice". Endocrinology. 156 (8): 2753–61. doi:10.1210/en.2015-1213. PMC 4511140. PMID 26052898.
- Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, Case N, Xie Z, Sen B, Romaine A, Pagnotti GM, Rubin CT, Styner MA, Horowitz MC, Rubin J (July 2014). "Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise". Bone. 64: 39–46. doi:10.1016/j.bone.2014.03.044. PMC 4041821. PMID 24709686.
- Coskun H, Summerfield TL, Kniss DA, Friedman A (2010). "Mathematical modeling of preadipocyte fate determination". Journal of Theoretical Biology. 265 (1): 87–94. doi:10.1016/j.jtbi.2010.03.047. PMID 20385145. Lay summary – ScienceDaily.
- Coskun H, Summerfield TL, Kniss DA, Friedman A (July 2010). "Mathematical modeling of preadipocyte fate determination". Journal of Theoretical Biology. 265 (1): 87–94. doi:10.1016/j.jtbi.2010.03.047. PMID 20385145.
- Fried SK, Lee MJ, Karastergiou K (July 2015). "Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology". Obesity (Review). 23 (7): 1345–52. doi:10.1002/oby.21133. PMC 4687449. PMID 26054752.
- Hong R, Choi DY, Do NY, Lim SC (July 2008). "Fine-needle aspiration cytology of a lipoblastoma: a case report". Diagnostic Cytopathology. 36 (7): 508–11. doi:10.1002/dc.20826. PMID 18528880.
- Ding H, Zheng S, Garcia-Ruiz D, Hou D, Wei Z, Liao Z, et al. (May 2016). "Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16". Nature Communications. 7: 11533. doi:10.1038/ncomms11533. PMC 4895052. PMID 27240637.
- Warden CH, Fisler JS (April 2008). "Comparisons of diets used in animal models of high-fat feeding". Cell Metabolism. 7 (4): 277. doi:10.1016/j.cmet.2008.03.014. PMC 2394560. PMID 18396128.
Regular chow is composed of agricultural byproducts, such as ground wheat, corn, or oats, alfalfa and soybean meals, a protein source such as fish, and vegetable oil and is supplemented with minerals and vitamins. Thus, chow is a high fiber diet containing complex carbohydrates, with fats from a variety of vegetable sources. Chow is inexpensive to manufacture and is palatable to rodents.
- Faust IM, Johnson PR, Stern JS, Hirsch J (September 1978). "Diet-induced adipocyte number increase in adult rats: a new model of obesity". The American Journal of Physiology. 235 (3): E279–86. doi:10.1152/ajpendo.1978.235.3.E279. PMID 696822.
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P (June 2008). "Dynamics of fat cell turnover in humans". Nature. 453 (7196): 783–7. doi:10.1038/nature06902. PMID 18454136.
- Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD (October 2010). "Regional differences in cellular mechanisms of adipose tissue gain with overfeeding". Proceedings of the National Academy of Sciences of the United States of America. 107 (42): 18226–31. doi:10.1073/pnas.1005259107. PMC 2964201. PMID 20921416.
- Blüher M (June 2009). "Adipose tissue dysfunction in obesity". Experimental and Clinical Endocrinology & Diabetes. 117 (6): 241–50. doi:10.1055/s-0029-1192044. PMID 19358089.
- Kahn SE, Hull RL, Utzschneider KM (December 2006). "Mechanisms linking obesity to insulin resistance and type 2 diabetes". Nature. 444 (7121): 840–6. doi:10.1038/nature05482. PMID 17167471.
- Kahn BB, Flier JS (August 2000). "Obesity and insulin resistance". The Journal of Clinical Investigation. 106 (4): 473–81. doi:10.1172/JCI10842. PMC 380258. PMID 10953022.
- Rawson RB (August 2003). "The SREBP pathway--insights from Insigs and insects". Nature Reviews Molecular Cell Biology. 4 (8): 631–40. doi:10.1038/nrm1174. PMID 12923525.
- Carobbio S, Hagen RM, Lelliott CJ, Slawik M, Medina-Gomez G, Tan CY, et al. (November 2013). "Adaptive changes of the Insig1/SREBP1/SCD1 set point help adipose tissue to cope with increased storage demands of obesity". Diabetes. 62 (11): 3697–708. doi:10.2337/db12-1748. PMC 3806615. PMID 23919961.
- Nelson LR, Bulun SE (September 2001). "Estrogen production and action". Journal of the American Academy of Dermatology. 45 (3 Suppl): S116–24. doi:10.1067/mjd.2001.117432. PMID 11511861.
- "FERTILITY FACT: Female Risks". American Society for Reproductive Medicine (ASRM). Archived from the original on 22 September 2007.
- Klok MD, Jakobsdottir S, Drent ML (January 2007). "The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review". Obesity Reviews. 8 (1): 21–34. doi:10.1111/j.1467-789X.2006.00270.x. PMID 17212793.
External links
| Wikimedia Commons has media related to Adipocytes. |
- Histology image: 08201loa – Histology Learning System at Boston University – "Connective Tissue: unilocular (white) adipocytes "
- Histology image: 04901lob – Histology Learning System at Boston University – "Connective Tissue: multilocular (brown) adipocytes"