Drostanolone
Drostanolone, or dromostanolone, is an anabolic–androgenic steroid (AAS) of the dihydrotestosterone (DHT) group which was never marketed.[1][2][3] An androgen ester prodrug of drostanolone, drostanolone propionate, was formerly used in the treatment of breast cancer in women under brand names such as Drolban, Masteril, and Masteron.[1][2][3][4] This ester has also been used non-medically for physique- or performance-enhancing purposes.[3]
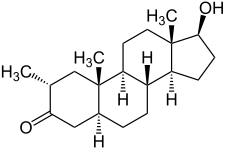 | |
| Clinical data | |
|---|---|
| Trade names | Drolban, Masteril, Masteron, others (all as drostanolone propionate) |
| Other names | Dromostanolone; 2α-Methyl-4,5α-dihydrotestosterone; 2α-Methyl-DHT; 2α-Methyl-5α-androstan-17β-ol-3-one |
| Routes of administration | Intramuscular injection (as drostanolone propionate) |
| Drug class | Androgen; Anabolic steroid |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.334 |
| Chemical and physical data | |
| Formula | C20H32O2 |
| Molar mass | 304.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Like other AAS, drostanolone is an agonist of the androgen receptor (AR).[3] It is not a substrate for 5α-reductase and is a poor substrate for 3α-hydroxysteroid dehydrogenase (3α-HSD), and therefore shows a high ratio of anabolic to androgenic activity.[3] As a DHT derivative, drostanolone is not a substrate for aromatase and hence cannot be aromatized into estrogenic metabolites.[3] While no data are available on the progestogenic activity of drostanolone, it is thought to have low or no such activity similarly to other DHT derivatives.[3] Since the drug is not 17α-alkylated, it is not known to cause hepatotoxicity.[3]
Chemistry
Drostanolone, also known as 2α-methyl-5α-dihydrotestosterone (2α-methyl-DHT) or as 2α-methyl-5α-androstan-17β-ol-3-one, is a synthetic androstane steroid and a derivative of DHT.[1][2][3] It is specifically DHT with a methyl group at the C2α position.[1][2][3]
History
Drostanolone and its ester drostanolone propionate were first described in 1959.[3][5] Drostanolone propionate was first introduced for medical use in 1961.[6]
Society and culture
Generic names
Drostanolone is the generic name of the drug and its INN, BAN, and DCF.[1][2] It has also been referred to as dromostanolone.[1][2]
Legal status
Drostanolone, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.[7]
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 652–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 377–. ISBN 978-3-88763-075-1.
- Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 517–. ISBN 978-0-9828280-1-4.
- Bennett MB, Helman P, Palmer P (November 1975). "Hormonal therapy of breast cancer with special reference to Masteril therapy". South African Medical Journal = Suid-Afrikaanse Tydskrif vir Geneeskunde. 49 (49): 2036–40. PMID 1242823.
- Ringold HJ, Batres E, Halpern O, Necoechea E (1959). "Steroids. CV.12-Methyl and 2-Hydroxymethylene-androstane Derivatives". Journal of the American Chemical Society. 81 (2): 427–432. doi:10.1021/ja01511a040. ISSN 0002-7863.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1402–. ISBN 978-0-8155-1856-3.
- Karch SB (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.