Cichlid
Cichlids /ˈsɪklɪdz/[1] are fish from the family Cichlidae in the order Cichliformes. Cichlids were traditionally classed in a suborder, Labroidei, along with the wrasses (Labridae), in the order Perciformes[2] but molecular studies have contradicted this grouping.[3] The closest living relatives of cichlids are probably the convict blennies and both families are classified in the 5th edition of Fishes of the World as the two families in the Cichliformes, part of the subseries Ovalentaria.[4] This family is both large and diverse. At least 1,650 species have been scientifically described,[5] making it one of the largest vertebrate families. New species are discovered annually, and many species remain undescribed. The actual number of species is therefore unknown, with estimates varying between 2,000 and 3,000.[6]
| Cichlid Temporal range: Eocene to present(molecular clock suggests Cretaceous origins) | |
|---|---|
 | |
| Common freshwater angelfish, Pterophyllum scalare | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Clade: | Percomorpha |
| (unranked): | Ovalentaria |
| Order: | Cichliformes |
| Family: | Cichlidae Bonaparte, 1835 |
| Subfamilies | |
|
Cichlinae | |
Many cichlids, particularly tilapia, are important food fishes, while others, such as the Cichla species, are valued game fish. The family also includes many popular freshwater aquarium fish kept by hobbyists, including the angelfish, oscars, and discus.[7][8] Cichlids have the largest number of endangered species among vertebrate families, most in the haplochromine group.[9] Cichlids are particularly well known for having evolved rapidly into many closely related but morphologically diverse species within large lakes, particularly Tanganyika, Victoria, Malawi, and Edward.[10][11] Their diversity in the African Great Lakes is important for the study of speciation in evolution.[12] Many cichlids introduced into waters outside of their natural range have become nuisances.[13]
All cichlids have some form of parental care for their eggs and fry. That parental care may come in the form of guarding the eggs and fry or it may come in the form of mouthbrooding.
Anatomy and appearance

Cichlids span a wide range of body sizes, from species as small as 2.5 cm (0.98 in) in length (e.g., female Neolamprologus multifasciatus) to much larger species approaching 1 m (3.3 ft) in length (Boulengerochromis and Cichla). As a group, cichlids exhibit a similar diversity of body shapes, ranging from strongly laterally compressed species (such as Altolamprologus, Pterophyllum, and Symphysodon) to species that are cylindrical and highly elongated (such as Julidochromis, Teleogramma, Teleocichla, Crenicichla, and Gobiocichla).[7] Generally, however, cichlids tend to be of medium size, ovate in shape, and slightly laterally compressed, and generally similar to the North American sunfishes in morphology, behavior, and ecology.[14]
Cichlids share a single key trait: the fusion of the lower pharyngeal bones into a single tooth-bearing structure. A complex set of muscles allows the upper and lower pharyngeal bones to be used as a second set of jaws for processing food, allowing a division of labor between the "true jaws" (mandibles) and the "pharyngeal jaws". Cichlids are efficient and often highly specialized feeders that capture and process a very wide variety of food items. This is assumed to be one reason why they are so diverse.[7]
The features that distinguish them from the other families in Labroidei include:[15]
- A single nostril on each side of the forehead, instead of two
- No bony shelf below the orbit of the eye
- Division of the lateral line organ into two sections, one on the upper half of the flank and a second along the midline of the flank from about halfway along the body to the base of the tail (except for genera Teleogramma and Gobiocichla)
- A distinctively shaped otolith
- The small intestine's left-side exit from the stomach instead of its right side as in other Labroidei
Taxonomy
Kullander (1998) recognizes eight subfamilies of cichlids: the Astronotinae, Cichlasomatinae, Cichlinae, Etroplinae, Geophaginae, Heterochromidinae, Pseudocrenilabrinae, and Retroculinae.[16] A ninth subfamily, Ptychochrominae, was later recognized by Sparks and Smith.[17] Cichlid taxonomy is still debated, and classification of genera cannot yet be definitively given. A comprehensive system of assigning species to monophyletic genera is still lacking, and there is not complete agreement on what genera should be recognized in this family.[18]
As an example of the classification problems, Kullander[19] placed the African genus Heterochromis phylogenetically within Neotropical cichlids, although later papers concluded otherwise. Other problems center upon the identity of the putative common ancestor for the Lake Victoria superflock (many closely related species sharing a single habitat), and the ancestral lineages of Tanganyikan cichlids.
Comparisons[20] between a morphologically based phylogeny[21] and analyses of gene loci[22] produce differences at the genus level. There remains a consensus that the Cichlidae as a family is monophyletic.[23][24]
In cichlid taxonomy, dentition was formerly used as a classifying characteristic. However, this was complicated by the fact that in many cichlids, tooth shape changes with age, due to wear, and cannot be relied upon. Genome sequencing and other technologies transformed cichlid taxonomy.[25]
Distribution and habitat

Cichlids are one of the largest vertebrate families in the world. They are most diverse in Africa and South America. Africa alone is estimated to host at least 1,600 species.[18] Central America and Mexico have about 120 species, as far north as the Rio Grande in southern Texas. Madagascar has its own distinctive species (Katria, Oxylapia, Paratilapia, Paretroplus, Ptychochromis, and Ptychochromoides), only distantly related to those on the African mainland.[15][27] Native cichlids are largely absent in Asia, except for 9 species in Israel, Lebanon, and Syria (Astatotilapia flaviijosephi, Oreochromis aureus, O. niloticus, Sarotherodon galilaeus, Coptodon zillii, and Tristramella spp.), two in Iran (Iranocichla), and three in India and Sri Lanka (Etroplus and Pseudetroplus).[18] If disregarding Trinidad and Tobago (where the few native cichlids are members of genera that are widespread in the South American mainland), the three species from the genus Nandopsis are the only cichlids from the Antilles in the Caribbean, specifically Cuba and Hispaniola. Europe, Australia, Antarctica, and North America north of the Rio Grande drainage have no native cichlids, although in Florida, Hawaii, Japan, northern Australia and elsewhere, feral populations of cichlids have become established as exotics.[26][28][29][30][31][32][33]
Although most cichlids are found at relatively shallow depths, several exceptions do exist. The deepest known occurrence are Trematocara at more than 300 m (980 ft) below the surface in Lake Tanganyika.[34] Others found in relatively deep waters include species such as Alticorpus macrocleithrum and Pallidochromis tokolosh down to 150 m (490 ft) below the surface in Lake Malawi,[35][36] and the whitish (nonpigmented) and blind Lamprologus lethops, which is believed to live as deep as 160 m (520 ft) below the surface in the Congo River.[37]
Cichlids are less commonly found in brackish and saltwater habitats, though many species tolerate brackish water for extended periods; Mayaheros urophthalmus, for example, is equally at home in freshwater marshes and mangrove swamps, and lives and breeds in saltwater environments such as the mangrove belts around barrier islands.[7] Several species of Tilapia, Sarotherodon, and Oreochromis are euryhaline and can disperse along brackish coastlines between rivers.[18] Only a few cichlids, however, inhabit primarily brackish or salt water, most notably Etroplus maculatus, Etroplus suratensis, and Sarotherodon melanotheron.[38] The perhaps most extreme habitats for cichlids are the warm hypersaline lakes where the members of the genera Alcolapia and Danakilia are found. Lake Abaeded in Eritrea encompasses the entire distribution of D. dinicolai, and its temperature ranges from 29 to 45 °C (84 to 113 °F).[39]
With the exception of the species from Cuba, Hispaniola, and Madagascar, cichlids have not reached any oceanic island and have a predominantly Gondwanan distribution, showing the precise sister relationships predicted by vicariance: Africa-South America and India-Madagascar.[40] The dispersal hypothesis, in contrast, requires cichlids to have negotiated thousands of kilometers of open ocean between India and Madagascar without colonizing any other island or, for that matter, crossing the Mozambique Channel to Africa. Although the vast majority of Malagasy cichlids are entirely restricted to fresh water, Ptychochromis grandidieri and Paretroplus polyactis are commonly found in coastal brackish water and they are apparently salt tolerant,[41][42] as is also the case for Etroplus maculatus and E. suratensis from India and Sri Lanka.[43][44]
Ecology
Feeding
Within the cichlid family, there are carnivores, herbivores, omnivores, planktivores, and detritivores, meaning Cichlidae encompasses essentially the full range of food consumption possible in the animal kingdom. Various species have morphological adaptations for specific food sources,[45] but most cichlids will consume a wider variety of foods based on availability. Carnivorous cichlids can be further divided into piscivorous and molluscivorous, since the morphology and hunting behavior differs greatly between the two categories. Piscivorous cichlids will eat other fish, fry, larvae, and eggs. Some species eat the offspring of mouthbrooders by head-ramming, wherein the hunter shoves its head into the mouth of a female in order to expel her young and eat them.[46] Molluscivorous cichlids have several hunting strategies amongst the varieties within the group. Lake Malawi cichlids consume substrate and filter it out through their gill rakers to eat the mollusks that were in the substrate. Gill rakers are finger-like structures that line the gills of some fish to catch any food that might escape through their gills.[47]
Many cichlids are primarily herbivores, feeding on algae (e.g. Petrochromis) and plants (e.g. Etroplus suratensis). Small animals, particularly invertebrates, are only a minor part of their diets.
Other cichlids are detritivores and eat organic material, called Aufwuchs; among these species are the tilapiines of the genera Oreochromis, Sarotherodon, and Tilapia.
Other cichlids are predatory and eat little or no plant matter. These include generalists that catch a variety of small animals, including other fishes and insect larvae (e.g. Pterophyllum), as well as variety of specialists. Trematocranus is a specialized snail-eater, while Pungu maclareni feeds on sponges. A number of cichlids feed on other fish, either entirely or in part. Crenicichla species are stealth-predators that lunge from concealment at passing small fish, while Rhamphochromis species are open-water pursuit predators that chase down their prey.[49] Paedophagous cichlids such as the Caprichromis species eat other species' eggs or young, in some cases ramming the heads of mouthbrooding species to force them to disgorge their young.[50][51][52][53] Among the more unusual feeding strategies are those of Corematodus, Docimodus evelynae, Plecodus, Perissodus, and Genyochromis spp., which feed on scales and fins of other fishes, a behavior known as lepidophagy,[54][55][56] along with the death-mimicking behaviour of Nimbochromis and Parachromis species, which lay motionless, luring small fish to their side prior to ambush.[57][58]
This variety of feeding styles has helped cichlids to inhabit similarly varied habitats. Its pharyngeal teeth (teeth in the throat) afford cichlids so many "niche" feeding strategies, because the jaws pick and hold food, while the pharyngeal teeth crush the prey.
Behavior
Aggression
Aggressive behavior in cichlids is ritualized and consists of multiple displays used to seek confrontation while being involved in evaluation of competitors,[59] coinciding with temporal proximity to mating. Displays of ritualized aggression in cichlids include a remarkably rapid change in coloration, during which a successfully dominant[59] territorial male assumes a more vivid and brighter coloration while a subordinate or "non-territorial" male assumes a dull-pale coloration.[60] In addition to color displays, cichlids employ their lateral lines to sense movements of water around their opponents to evaluate the competing male for physical traits/fitness.[61] Male cichlids are very territorial due to the pressure of reproduction, males establish their territory and social status by physically driving out[62] challenging males (novel intruders)[63] through lateral displays (parallel orientation, uncovering gills),[64] biting, or mouth fights (head-on collisions of ajar mouths, measuring jaw sizes, and biting each other's jaws). The cichlid social dichotomy is composed of a single dominant with multiple subordinates, where the physical aggression of males become a contest for resources[62] (mates, territory, food). Female cichlids prefer to mate with a successfully alpha male with vivid coloration, whose territory has food readily available.
Mating
Cichlids mate either monogamously or polygamously.[7] The mating system of a given cichlid species is not consistently associated with its brooding system. For example, although most monogamous cichlids are not mouthbrooders, Chromidotilapia, Gymnogeophagus, Spathodus and Tanganicodus all include – or consist entirely of – monogamous mouthbrooders. In contrast, numerous open- or cave-spawning cichlids are polygamous; examples include many Apistogramma, Lamprologus, Nannacara, and Pelvicachromis species.[7][65]
Most adult male cichlids, specifically in the Haplochromini tribe of cichlids, exhibit a unique pattern of oval-shaped, color dots on their anal fins. These phenomena are known as egg-spots and aid in the mouthbrooding mechanisms of cichlids. The egg-spots consist of carotenoid-based pigment cells, which indicates a high cost to the organism, when considering that fish are not able to synthesize their own carotenoids.[66]
The mimicry of egg-spots is used by males for the fertilization process. Mouthbrooding females lay eggs and immediately snatch them up with their mouths. Over millions of years, male cichlids have evolved egg-spots to initiate the fertilization process more efficiently.[67] When the females are snatching up the eggs into their mouth, the males gyrate their anal fins, which illuminates the egg-spots on his tail. Afterwards, the female, believing these are her eggs, places her mouth to the anal fin (specifically the genital papilla) of the male, which is when he discharges sperm into her mouth and fertilizes the eggs.[66]
The genuine color of egg spots is a yellow, red or orange inner circle with a colorless ring surrounding the shape. Through phylogenetic analysis, using the mitochondrial ND2 gene, it was hypothesized that the true egg spots evolved in the common ancestor of the Astatoreochromis-lineage and the modern Haplochrominis. This ancestor was most likely riverine in origin, based on the most parsimonious representation of habitat type in the cichlid family.[68] The presence of egg-spots in a turbid riverine environment, would seem particularly beneficial and necessary for intra-species communication.[68]
There are two pigmentation genes that are found to be associated with egg-spot patterning and color arrangement. These are fhl2-a and fhl2-b, which are paralogs.[67] These genes aid in pattern formation and cell-fate determination in early embryonic development. The highest expression of these genes was temporally correlated with egg-spot formation. A SINE (short interspersed repetitive element) was also seen to be associated with egg-spots. Specifically, it was evident upstream of the transcriptional start site of fhl2 in only haplochrominis species with egg-spots[67]
Brood care
Pit-spawning in cichlids
Pit-spawning, also referred to as substrate breeding, is a behavior in cichlid fish in which a fish will build a pit in the sand or ground where they will court their mate and consequently spawn with them.[69] There are many different factors that go into this behavior of pit-spawning including female choice of the male and pit size, as well as the male defense of the pits once they are dug in the sand.[70] Cichlids are often divided into two main groups: mouthbrooders and substrate brooders. There are different parenting investment levels and behaviors associated with each type of reproduction.[71] As pit-spawning is a reproductive behavior, there are many different physiological changes that occur in the cichlid while this process is occurring that interferes with social interaction.[72] There are different kinds of species that pit-spawn and many different morphological changes that occur because of this behavioral experience that has been studied in cichlids.[69] Pit-spawning is an evolved behavior across the cichlid group. There is phylogenetic evidence from cichlids in Lake Tanganyika that could be helpful in uncovering the evolution of their reproductive behaviors.[73] There are several important behaviors that are associated with pit-spawning, including parental care, food provisioning,[74] and brood guarding.[75]
Mouthbrooders vs. pit-spawning
One of the differences studied in African cichlids is the difference in their reproductive behavior. There are some species who pit-spawn and some who are known as mouthbrooders. Mouthbrooding is a reproduction technique where the fish will scoop up eggs and fry for protection.[76] While this reproductive behavior differs from species to species in the details, the general basis of the behavior is the same. This differs completely from the other reproductive behavior of cichlids, pit-spawning. As stated in the introduction, pit-spawning is when cichlids dig pits for their eggs rather than holding them in their mouths. These two types of behavior differ not only in the way that they care for their eggs and fry but also how they choose their mates and breeding grounds. In a 1995 study, Nelson found that in pit-spawning females will choose with which males to mate based on the size of the pit that they dig as well as some of the physical characteristics shown in the males.[77] Pit-spawning also differs from mouthbrooding in the size and post-natal care that is exhibited. Eggs that have been hatched from pit-spawning cichlids are usually smaller than those of mouthbrooders. Pit-spawners' eggs are usually around 2 mm while mouthbrooders are typically around 7 mm. While there are different behaviors that take place -post-natally between mouthbrooders and pit-spawners, there are some similarities. Something that both mouthbrooders and pit-spawning cichlids have in common is that the female in both cases will take care of their young after they are hatched. In some cases, there is biparental care, discussed in a later section, but the female will always take an interest in caring for the eggs and newly hatched fry.[78]
How pit-spawning is done
There are many different species of cichlid that use pit-spawning as reproductive behavior, but one of the less commonly studied species that exhibit this behavior is the Neotropical cichlid fish or the Cichlasoma dimerus.[69] This fish is a substrate-breeder who displays biparental care after the fish have hatched from their eggs. In a study done by Alonso, et al., they studied the reproductive behavior as well as the social behavior of this particular species to see how they accomplished their pit-spawning as well as behaviors associated with the social aspect of these animals. These researchers looked at a variety of different physiological factors in the cichlids including hormone levels, color changes, plasma cortisol levels, as well as their behavior directly associated with pit-spawning. They found that the entire spawning process could take about ninety minutes and anywhere between 400 and 800 eggs could be laid in the process. The female would deposit ~ten eggs at a time, attaching them to the spawning surface which may be a pit constructed on the substrate or another surface. They found that the number of eggs that were laid correlated to the amount of space that they had on the substrate. Once the eggs were attached, the male would swim over the eggs and fertilize them. The parents would then dig pits in the sand, 10–20 cm wide and 5–10 cm deep where larvae would be transferred after hatching. Larvae would begin swimming eight days after fertilization and parenting behaviors, as well as some of the physiological factors measured, would change.[69]
Color changes
In Alonso's study, they noted that color changes were present before and after the pit-spawning occurred. For example, after the larvae were transferred and the pits were beginning to be protected, their fins turned a dark grey color.[69] In another study, done by Brown and Marshall, they studied the Rainbow Cichlid, Herotilapia multispinosa.[72] In this study, they looked at the color change that occurs in this fish throughout the spawning process. They found that pre-spawning, the Rainbow Cichlid was an olive color with grey bands. Once pre-spawning behaviors started, the body and fins of the fish became more of a gold color. When the eggs are finished being laid, the pelvic fin all the way back to the caudal fin turns to a darker color and blackens in both the males and the females.[72]
Pit sizes
As mentioned previously, a study done by Nelson shows that females prefer a bigger pit size when choosing where to lay eggs.[77] Another study done by York, et al., shows that there are differences in the sizes of pits that pit-spawning cichlids create, as well as a change in the morphology of the pits.[79] They found that there may be evolutionary differences between species of fish that cause them to either create pits or castles when spawning. The differences that they found were changes in the way that each species fed, their macrohabitat, and the ability of their sensory systems.[79]
Evolution
Cichlids are renowned for their recent, rapid evolutionary radiation, both across the entire clade and within different communities across separate habitats.[71][73][79][80][81][82] Within their phylogeny, there have been many parallel instances of lineages evolving to the same trait and multiple cases of reversion to an ancestral trait. The Cichlidae family arose between 80 and 100 million years ago within the order Perciformes (perch-like fishes).[83] Cichlidae can be split into a few groups based on their geographic location: Madagascar, Indian, African, and Neotropical (or South American). The most famous and diverse group, the African cichlids, can be further split either into Eastern and Western varieties, or into groups depending on which lake the species is from: Lake Malawi, Lake Victoria, or Lake Tanganyika.[83][81] Of these subgroups, the Madagascar and Indian cichlids are the most basal and least diverse.[10] Of the African cichlids, the West African or Lake Tanganyika cichlids are the most basal.[73][83] Cichlids' common ancestor is believed to have been a spit-spawning species.[81] Both Madagascar and Indian cichlids retain this feature. However, of the African cichlids, all extant substrate brooding species originate solely from Lake Tanganyika.[71][81] The ancestor of the Lake Malawi and Lake Victoria cichlids were mouthbrooders. Similarly, only around 30% of South American cichlids are thought to retain the ancestral substrate-brooding trait. Mouthbrooding is thought to have evolved individually up to 14 times, and a return to substrate brooding as many as 3 separate times between both African and Neotropical species.[81]
Associated behaviors
Cichlids have a great variety of behaviors associated with substrate-brooding, including courtship and parental care alongside the brooding and nest-building behaviors needed for pit-spawning. Cichlids behavior typically revolves around establishing and defending territories when not courting, brooding, or raising young. Encounters between males and males or females and females result are agonistic, while an encounter between a male and female leads to courtship.[84] Courtship in male cichlids follows the establishment of some form of territory, sometimes coupled with building a bower to attract mates.[70][79][84] After this, male may attempt to attract female cichlids to their territories via a variety of lekking display strategies or otherwise seek out females of their species.[70] However, cichlids, at the time of spawning, undergo a behavioral change such that they become less receptive to outside interactions.[84] This is often coupled with some physiological change in appearance.[69][72][84]
Brood care
Cichlids can have maternal, paternal, or biparental care. Maternal care is most common among mouth-brooders, however cichlids' common ancestor is thought to exhibit paternal-only care.[81] Other individuals outside of the parents may also play a role in raising young; in the biparental daffodil cichlid (Neolamprologus pulcher), closely related satellite males, those males who surround other males territories and attempt to mate with female cichlids in the area, help rear the primary male's offspring as well as their own.[85] A common form of brood care involves food provisioning. For example, female Lyretail cichlids (Neolamprologus modabu) will dig at sandy substrate more to push nutritional detritus and zooplankton into the surrounding water. Adult N. modabu perform this strategy to collect food for themselves, however will dig more when offspring are present, likely to feed their fry.[75][86] This substrate-disruption strategy is rather common and can also be seen in Convict cichlids (Cichlasoma nigrofasciatum).[74][86] Other cichlids have an ectothermal mucous that they grow and feed to their young, while still others chew and distribute caught food to offspring. These strategies, however, are less common in pit-spawning cichlids.[86]
See also
Mouthbrooders, Cichlids, Parental care

Cichlids have highly organized breeding activities.[18] All species show some form of parental care for both eggs and larvae, often nurturing free-swimming young until they are weeks or months old. Communal parental care, where multiple monogamous pairs care for a mixed school of young have also been observed in multiple cichlid species, including Amphilophus citrinellus, Etroplus suratensis, and Tilapia rendalli.[87][88][89] Comparably, the fry of Neolamprologus brichardi, a species that commonly lives in large groups, are protected not only by the adults, but also by older juveniles from previous spawns.[90] Several cichlids, including discus (Symphysodon spp.), some Amphilophus species, Etroplus, and Uaru species, feed their young with a skin secretion from mucous glands.[7][91]
The species Neolamprologus pulcher uses a cooperative breeding system, in which one breeding pair has many helpers which are subordinate to the dominant breeders.
Parental care falls into one of four categories:[91] substrate or open brooders, secretive cave brooders (also known as guarding speleophils[92]), and at least two types of mouthbrooders, ovophile mouthbrooders and larvophile mouthbrooders.[93]
Open brooding
Open- or substrate-brooding cichlids lay their eggs in the open, on rocks, leaves, or logs. Examples of open-brooding cichlids include Pterophyllum and Symphysodon species and Anomalochromis thomasi. Male and female parents usually engage in differing brooding roles. Most commonly, the male patrols the pair's territory and repels intruders, while the female fans water over the eggs, removing the infertile and leading the fry while foraging. However, both sexes are able to perform the full range of parenting behaviours.[93]
Cave brooding

Secretive cave-spawning cichlids lay their eggs in caves, crevices, holes, or discarded mollusc shells, frequently attaching the eggs to the roof of the chamber. Examples include Pelvicachromis spp., Archocentrus spp., and Apistogramma spp.[91] Free-swimming fry and parents communicate in captivity and in the wild. Frequently, this communication is based on body movements, such as shaking and pelvic fin flicking. In addition, open- and cave-brooding parents assist in finding food resources for their fry. Multiple neotropical cichlid species perform leaf-turning and fin-digging behaviors.[93]
Ovophile mouthbrooding
Ovophile mouthbrooders incubate their eggs in their mouths as soon as they are laid, and frequently mouthbrood free-swimming fry for several weeks. Examples include many East African Rift lakes (Lake Malawi, Lake Tanganyika and Lake Victoria) endemics, e.g.: Maylandia, Pseudotropheus, Tropheus, and Astatotilapia burtoni, along with some South American cichlids such as Geophagus steindachneri.
Larvophile mouthbrooding
Larvophile mouthbrooders lay eggs in the open or in a cave and take the hatched larvae into the mouth. Examples include some variants of Geophagus altifrons, and some Aequidens, Gymnogeophagus, and Satanoperca, as well as Oreochromis mossambicus and Oreochromis niloticus.[7][91] Mouthbrooders, whether of eggs or larvae, are predominantly females. Exceptions that also involve the males include eretmodine cichlids (genera Spathodus, Eretmodus, and Tanganicodus), some Sarotherodon species (such as Sarotherodon melanotheron[94]), Chromidotilapia guentheri, and some Aequidens species.[7][93][95] This method appears to have evolved independently in several groups of African cichlids.[18]
Speciation
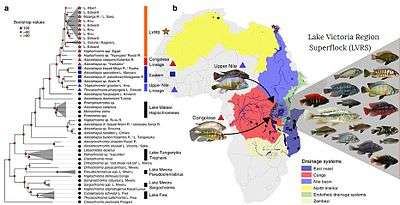
Cichlids provide scientists with a unique perspective of speciation, having become extremely diverse in the more recent geological past. It is widely believed that one of the contributing factors to their diversification are the various forms of prey processing displayed by cichlid pharyngeal jaw apparatus. These different jaw apparatus allow for a broad range of feeding strategies including: algae scraping, snail crushing, planktivores, piscivores, and insectivores.[97] Some cichlids can also show phenotypic plasticity in their pharyngeal jaws, which can also help lead to speciation. In response to different diets or food scarcity, members of the same species can display different jaw morphologies that are better suited to different feeding strategies. As species members begin to concentrate around different food sources and continue their life cycle, they most likely spawn with like individuals. This can reinforce the jaw morphology and given enough time, create new species.[98] Such a process can happen through allopatric speciation, whereby species diverge according to different selection pressures in different geographical areas, or through sympatric speciation, by which new species evolve from a common ancestor while remaining in the same area. In Lake Apoyo in Nicaragua, Amphilophus zaliosus and its sister species Amphilophus citrinellus display many of the criteria needed for sympatric speciation.[99] In the African rift lake system, cichlid species in numerous distinct lakes evolved from a shared hybrid swarm.[96]
Population status
In 2010, the International Union for Conservation of Nature classified 184 species as vulnerable, 52 as endangered, and 106 as critically endangered.[100] At present, the IUCN only lists Yssichromis sp. nov. "argens" as extinct in the wild, and six species are listed as entirely extinct, but it is acknowledged that many more possibly belong in these categories (for example, Haplochromis aelocephalus, H. apogonoides, H. dentex, H. dichrourus and numerous other members of the genus Haplochromis have not been seen since the 1980s, but are maintained as Critically Endangered in the small chance that tiny –but currently unknown– populations survive).[100]
Lake Victoria

Because of the introduced Nile perch (Lates niloticus), Nile tilapia (Oreochromis niloticus), and water hyacinth, deforestation that led to water siltation, and overfishing, many Lake Victoria cichlid species have become extinct or been drastically reduced. By around 1980, lake fisheries yielded only 1% cichlids, a drastic decline from 80% in earlier years.[102]
By far the largest Lake Victoria group are the haplochromine cichlids, with more than 500 species, but at least 200 of these (approximately 40%) have become extinct,[103][104][105] and many others are seriously threatened.[106] Initially it was feared that the percentage of extinct species was even higher,[107] but some species have been rediscovered after the Nile perch started to decline in the 1990s.[104][108] Some species have survived in nearby small satellite lakes,[108] or in refugia among rocks or papyrus sedges (protecting them from the Nile perch),[109] or have adapted to the human-induced changes in the lake itself.[104][105] The species were often specialists and these were not affected to the same extent. For example, the piscivorous haplochromines were particularly hard hit with a high number of extinctions,[110] while the zooplanktivorous haplochromines reached densities in 2001 that were similar to before the drastic decline, although consisting of fewer species and with some changes in their ecology.[104]
Food and game fish
Although cichlids are mostly small- to medium-sized, many are notable as food and game fishes. With few thick rib bones and tasty flesh, artisan fishing is not uncommon in Central America and South America, as well as areas surrounding the African rift lakes.[102]
Tilapia
The most important food cichlids, however, are the tilapiines of North Africa. Fast growing, tolerant of stocking density, and adaptable, tilapiine species have been introduced and farmed extensively in many parts of Asia and are increasingly common aquaculture targets elsewhere.
Farmed tilapia production is about 1,500,000 t (1,500,000 long tons; 1,700,000 short tons) annually, with an estimated value of US$1.8 billion,[111] about equal to that of salmon and trout.
Unlike those carnivorous fish, tilapia can feed on algae or any plant-based food. This reduces the cost of tilapia farming, reduces fishing pressure on prey species, avoids concentrating toxins that accumulate at higher levels of the food chain, and makes tilapia the preferred "aquatic chickens" of the trade.[102]
Game fish
Many large cichlids are popular game fish. The peacock bass (Cichla species) of South America is one of the most popular sportfish. It was introduced in many waters around the world. In Florida, this fish generates millions of hours of fishing and sportfishing revenue of more than US$8 million a year.[112] Other cichlids preferred by anglers include the oscar, Mayan cichlid (Cichlasoma urophthalmus), and jaguar guapote (Parachromis managuensis).[112]
Aquarium fish

Since 1945, cichlids have become increasingly popular as aquarium fish.[7][91][93][113][114][115][116]
The most common species in hobbyist aquaria is Pterophyllum scalare from the Amazon River basin in tropical South America, known in the trade as the "angelfish". Other popular or readily available species include the oscar (Astronotus ocellatus), convict cichlid (Archocentrus nigrofasciatus) and discus fish (Symphysodon).[7]
Hybrids and selective breeding

Some cichlids readily hybridize with related species, both in the wild and under artificial conditions.[117] Other groups of fishes, such as European cyprinids, also hybridize.[118] Unusually, cichlid hybrids have been put to extensive commercial use, in particular for aquaculture and aquaria.[8][119] The hybrid red strain of tilapia, for example, is often preferred in aquaculture for its rapid growth. Tilapia hybridization can produce all-male populations to control stock density or prevent reproduction in ponds.[8]
Aquarium hybrids
The most common aquarium hybrid is perhaps the blood parrot cichlid, which is a cross of several species, especially from species in the genus Amphilophus. (There are many hypotheses, but the most likely is: Amphilophus labiatus x Vieja synspillus With a triangular-shaped mouth, an abnormal spine, and an occasionally missing caudal fin (known as the "love heart" parrot cichlid), the fish is controversial among aquarists. Some have called blood parrot cichlids "the Frankenstein monster of the fish world".[120] Another notable hybrid, the flowerhorn cichlid, was very popular in some parts of Asia from 2001 until late 2003, and is believed to bring good luck to its owner.[121] The popularity of the flowerhorn cichlid declined in 2004.[122] Owners released many specimens into the rivers and canals of Malaysia and Singapore, where they threaten endemic communities.[123]

Numerous cichlid species have been selectively bred to develop ornamental aquarium strains. The most intensive programs have involved angelfish and discus, and many mutations that affect both coloration and fins are known.[7][124][125] Other cichlids have been bred for albino, leucistic, and xanthistic pigment mutations, including oscars, convict cichlid and Pelvicachromis pulcher.[7][91] Both dominant and recessive pigment mutations have been observed.[126] In convict cichlids, for example, a leucistic coloration is recessively inherited,[127] while in Oreochromis niloticus niloticus, red coloration is caused by a dominant inherited mutation.[128]
This selective breeding may have unintended consequences. For example, hybrid strains of Mikrogeophagus ramirezi have health and fertility problems.[129] Similarly, intentional inbreeding can cause physical abnormalities, such as the notched phenotype in angelfish.[130]
Genera
The genus list is as per FishBase. Studies are continuing, however, on the members of this family, particularly the haplochromine cichlids of the African rift lakes.[15]
|
|
|
Images of cichlids
 The oscar (Astronotus ocellatus) is one of the most popular cichlids in the fishkeeping hobby.
The oscar (Astronotus ocellatus) is one of the most popular cichlids in the fishkeeping hobby. The butterfly peacock bass (Cichla ocellaris) was introduced intentionally in Florida as gamefish.
The butterfly peacock bass (Cichla ocellaris) was introduced intentionally in Florida as gamefish. The Nile tilapia (Oreochromis niloticus) is farmed extensively as food fish in many parts of the world.
The Nile tilapia (Oreochromis niloticus) is farmed extensively as food fish in many parts of the world. The angelfish (Pterophyllum scalare) has long been commercially bred for the aquarium trade.
The angelfish (Pterophyllum scalare) has long been commercially bred for the aquarium trade. Sexual dimorphism is common in cichlids. Shown here are a male (front, with egg spots) and a female (rear) Maylandia lombardoi.
Sexual dimorphism is common in cichlids. Shown here are a male (front, with egg spots) and a female (rear) Maylandia lombardoi. A pair of blue rams (Mikrogeophagus ramirezi), male in front, female behind. Many cichlids form strong pair bonds while breeding.
A pair of blue rams (Mikrogeophagus ramirezi), male in front, female behind. Many cichlids form strong pair bonds while breeding. A discus (Symphysodon spp.) is guarding its eggs. Advanced broodcare is one of the defining characteristics of cichlids.
A discus (Symphysodon spp.) is guarding its eggs. Advanced broodcare is one of the defining characteristics of cichlids. Lake Malawi, Eastern Africa, is home to numerous cichild species including this Livingston's cichlid (Nimbochromis livingstonii).
Lake Malawi, Eastern Africa, is home to numerous cichild species including this Livingston's cichlid (Nimbochromis livingstonii). Also from Lake Malawi
Also from Lake Malawi Also from Lake Malawi
Also from Lake Malawi
.jpg) The Texas cichlid (Herichthys cyanoguttatus) is the only cichlid native to the United States.
The Texas cichlid (Herichthys cyanoguttatus) is the only cichlid native to the United States..jpg) Pelvicachromis pulcher is a West African riverine cichlid, and part of the aquarists dwarf cichlid group.
Pelvicachromis pulcher is a West African riverine cichlid, and part of the aquarists dwarf cichlid group. The flowerhorn cichlid is a man-made hybrid that has recently gained popularity among aquarists, particularly in Asia.
The flowerhorn cichlid is a man-made hybrid that has recently gained popularity among aquarists, particularly in Asia. Ivanacara adoketa, a dwarf cichlid from Brazil
Ivanacara adoketa, a dwarf cichlid from Brazil The red terror cichlid is a highly aggressive species from the rivers of Northeast South America.
The red terror cichlid is a highly aggressive species from the rivers of Northeast South America. A juvenile female Maylandia lombardoi with faint stripes
A juvenile female Maylandia lombardoi with faint stripes A juvenile Aequidens diadema
A juvenile Aequidens diadema
References
- Cichlid is frequently mispronounced in the pet trade as if spelled "chicklid" /ˈtʃɪklɪd/, presumably from confusion with names like Chiclets, and with Italian words like cioppino and ciao that start with ci- and the sound /tʃ/.
- Stiassny, M.L.J.; Jensen, J.S. (1987). "Labroid intrarelationships revisited: morphological complexity, key innovations, and the study of comparative diversity". Bulletin of the Museum of Comparative Zoology. 151: 269–319.
- Wainwright, Peter C.; et al. (2012). "The Evolution of Pharyngognathy: A Phylogenetic and Functional Appraisal of the Pharyngeal Jaw Key Innovation in Labroid Fishes and Beyond". Systematic Biology. 61 (6): 1001–1027. doi:10.1093/sysbio/sys060. PMID 22744773.
- J. S. Nelson; T. C. Grande; M. V. H. Wilson (2016). Fishes of the World (5th ed.). Wiley. p. 752. ISBN 978-1-118-34233-6.
- "List of Nominal Species of Cichlidae, in Froese, Rainer, and Daniel Pauly, eds. (2012). FishBase". February 2012.
- Stiassny, M., G. G. Teugels & C. D. Hopkins (2007). The Fresh and Brackish Water Fishes of Lower Guinea, West-Central Africa – Vol. 2. Musée Royal de l'Afrique Centrale. p. 269. ISBN 978-90-74752-21-3.CS1 maint: multiple names: authors list (link)
- Loiselle, P.V. (1994). The Cichlid Aquarium. Tetra Press. ISBN 978-1-56465-146-4.
- Kosswig, Curt (June 1963). "Ways of Speciation in Fishes". Copeia. 1963 (2): 238–244. doi:10.2307/1441338. JSTOR 1441338.
- Reid, G. M. (December 1990). "Captive breeding for the conservation of cichlid fishes". Journal of Fish Biology. 37: 157–166. doi:10.1111/j.1095-8649.1990.tb05031.x.
- Salzburger W.; Mack T.; Verheyen E.; Meyer A. (2005). "Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes" (PDF). BMC Evolutionary Biology. 5 (17): 17. doi:10.1186/1471-2148-5-17. PMC 554777. PMID 15723698.
- Snoeks, J. (ed.) (2004). The cichlid diversity of Lake Malawi/Nyasa/Niassa: identification, distribution and taxonomy. Cichlid Press. ISBN 978-0-9668255-8-9.CS1 maint: extra text: authors list (link)
- Kornfield, Irv; Smith, Peter (November 2000). "African Cichlid Fishes: Model Systems for Evolutionary Biology". Annual Review of Ecology and Systematics. 31: 163–196. doi:10.1146/annurev.ecolsys.31.1.163.
- Gulf States Marine Fisheries Commission. "Fact sheet for Oreochromis mossambicus (Peters, 1852)". Gulf States Marine Fisheries Commission. Archived from the original on 18 August 2007. Retrieved 20 October 2006.
- Helfman G.; Collette B.; Facey D. (1997). The Diversity of Fishes. Blackwell Publishing, Inc. pp. 256–257. ISBN 978-0-86542-256-8.
- Froese, Rainer, and Daniel Pauly, eds. (2006). "Cichlidae" in FishBase. April 2006 version.
- Kullander, S.O. (1998). "A phylogeny and classification of the South American Cichlidae (Teleostei: Perciformes)". In L.R. Malabarba; R.E. Reis; R.P. Vari; Z.M. Lucena; C.A.S. Lucena (eds.). Phylogeny and classification of neotropical fishes. Porto Alegre: EDIPUCRS. pp. 461–498. ISBN 978-85-7430-035-1.
- Sparks, J.S.; Smith, W.L. (2004). "Phylogeny and biogeography of cichlid fishes (Teleostei: Perciformes: Cichlidae)". Cladistics. 20 (6): 501–517. CiteSeerX 10.1.1.595.2118. doi:10.1111/j.1096-0031.2004.00038.x.
- Nelson, Joseph, S. (2006). Fishes of the World. John Wiley & Sons, Inc. ISBN 978-0-471-25031-9.
- Phylogeny of major groups of cichlids
- Multilocus Phylogeny of Cichlid Fishes (Pisces: Perciformes): Evolutionary Comparison of Microsatellite and Single-Copy Nuclear Loci by Streelman, Zardoya, Meyer and Karl (1998) (Mol. Biol. Evol. 15(7):798–808. 1998, paper available as PDF here
- Stiassny, 1991
- maximum-parsimony bootstrap consensus trees and majority-rule trees and other similar phylogenetic trees
- From the various nuclear and mitochondrial DNA analyses in this and other papers
- Further insights into the attractiveness of Cichlid taxonomy as a fertile area of research is given by the paper The species flocks of East African cichlid fishes: recent advances in molecular phylogenetics and population genetics by Salzburger and Meyer (Naturwissenschaften (2004) 91:277–290, paper available as PDF here), in which the advances made in the analysis of the phylogeny of the Lake Victoria superflock (among other East African Cichlids) is discussed in depth.
- Highlighted by Dr Humphry Greenwood of the Natural History Museum, London, in a paper in 1977 (cited in TFH magazine, August 1977, with a follow up letter by Dr Greenwood in the November 1977 issue complaining about poor reportage of his work).
- Koehn, J.D.; MacKenzie, R.F. (2004). "Priority management actions for alien freshwater fish species in Australia" (PDF). New Zealand Journal of Marine and Freshwater Research. 38 (3): 457–472. doi:10.1080/00288330.2004.9517253. Archived from the original (PDF) on 28 October 2004. Retrieved 19 April 2007.
- Boruchowitz, D. E. (2006). Guide to Cichlids. T.F.H. Publications. ISBN 978-0-7938-0584-6.
- ABC Far North Queensland. "Tilapia :: Far North Queensland". Archived from the original on 17 October 2007. Retrieved 19 April 2007.
- Froese, R.; D. Pauly (eds.). "Archocentrus nigrofasciatus, Convict cichlid". FishBase. Archived from the original on 1 December 2008. Retrieved 29 March 2007.
- Yamamoto, M.N.; Tagawa, A.W. (2000). Hawai'i's native and exotic freshwater animals. Honolulu, Hawaii: Mutual Publishing. p. 200.
- Page, L.M.; Burr, B.M. (1991). A field guide to freshwater fishes of North America north of Mexico. Boston: Houghton Mifflin Company. pp. 432. ISBN 978-0-395-35307-3.
- University of Southern Mississippi/College of Marine Sciences/Gulf Coast Research Laboratory (3 August 2005). "Fact Sheet for Tilapia zilli (Gervais, 1848)". Gulf States Marine Fisheries Commission. Archived from the original on 18 August 2007. Retrieved 10 February 2007.
- Fuller, Pam L.; Leo G. Nico (11 October 2002). "Nonindigenous Fishes of Florida – With a Focus on South Florida". U.S. Department of the Interior, U.S. Geological Survey, Center for Coastal Geology. Retrieved 10 February 2007.
- Loiselle, Paul (1994). The Cichlid Aquarium, p. 304. Tetra Press, Germany. ISBN 978-1564651464.
- Froese, Rainer and Pauly, Daniel, eds. (2006). "Alticorpus macrocleithrum" in FishBase. April 2006 version.
- Froese, Rainer and Pauly, Daniel, eds. (2006). "Pallidochromis tokolosh" in FishBase. April 2006 version.
- Norlander, Britt (20 April 2009). Rough waters: one of the world's most turbulent rivers is home to a wide array of fish species. Now, large dams are threatening their future. Science World
- Frank Schäfer (2005). Brackish-Water Fishes. Aqualog. ISBN 978-3-936027-82-2.
- Stiassny, de Marchi & Lamboj (2010). A new species of Danakilia (Teleostei, Cichlidae) from Lake Abaeded in the Danakil Depression of Eritrea (East Africa). Zootaxa 2690: 43–52.
- Chakrabarty, P., Cichlid Biogeography: Comment and Review, Fish and Fisheries, Volume 5, Pages 97–119, 2004
- Stiassny, M., and Sparks, J. S. (2006). Phylogeny and Taxonomic Revision of the endemic Malagasy genus Ptychochromis (Teleostei: Cichlidae), with the description of five new species and a diagnosis for Katria, new genus. American Museum Novitates 3535.
- Sparks, J. S. (2008). Phylogeny of the Cichlid Subfamily Etroplinae and Taxonomic Revision of the Malagasy Cichlid Genus Paretroplus (Teleostei: Cichlidae). Bulletin of the American Museum of Natural History Number 314: 1–151
- Froese, Rainer and Pauly, Daniel, eds. (2006). "Etroplus maculatus" in FishBase. April 2006 version.
- Froese, Rainer and Pauly, Daniel, eds. (2006). "Etroplus suratensis" in FishBase. April 2006 version.
- Kullander, S.O. "Family Cichlidae-Cichlids". FishBase. Retrieved 4 December 2019.
- Jonna, R. Jamil. "Cichlidae". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 3 December 2019.
- De Iuliis, Gerardo (2011). The Disection of Vertebrates. Elsevier Inc. pp. 27–77. Retrieved 4 December 2019.
- Ribbink, A.J.; Lewis, D.S.C. (1982). "Melanochromis crabro sp. nov.: a cichlid fish from Lake Malawi which feeds on ectoparasites and catfish eggs". Netherlands Journal of Zoology. 32 (1): 72–87. doi:10.1163/002829682X00058..
- Oliver, M.K. (18 November 1999). "Rhamphochromis esox". malawicichlids.com: The Cichlid Fishes of Lake Malawi. Retrieved 19 April 2007.
- Ribbink, A.J.; Ribbink, A.C. (1997). "Paedophagia among cichlid fishes of Lake Victoria and Lake Malawi/Nyasa". South African Journal of Science. 93: 509–512.
- McKaye, K.R.; Kocher, T. (1983). "Head ramming behaviour by three paedophagous cichlids in Lake Malawi, Africa". Animal Behaviour. 31: 206–210. doi:10.1016/S0003-3472(83)80190-0.
- Wilhelm, W. (1980). "The disputed feeding behavior of a paedophagous haplochromine cichlid (Pisces) observed and discussed". Behaviour. 74 (3): 310–322. doi:10.1163/156853980X00528.
- Konings, A. (2007). "Paedophagy in Malawi cichlids". Cichlid News. 16: 28–32.
- Trewavas, E. (1947). "An example of "mimicry" in fishes". Nature. 160 (4056): 120. doi:10.1038/160120a0. PMID 20256157.
- Eccles, D.H.; D.S.C. Lewis (1976). "A taxonomic study of the genus Docimodus Boulenger (Pisces, Cichlidae) a group of fishes with unusual feeding habits from Lake Malawi". Zoological Journal of the Linnean Society. 58 (2): 165–172. doi:10.1111/j.1096-3642.1976.tb00826.x.
- Nshombo, M. (1991). "Occasional egg-eating by the scale-eater Plecodus straeleni (Cichlidae) of Lake Tanganyika". Environmental Biology of Fishes. 31 (2): 207–212. doi:10.1007/BF00001022.
- Tobler, M. (2005). "Feigning death in the Central American cichlid Parachromis friedrichsthalii". Journal of Fish Biology. 66 (3): 877–881. doi:10.1111/j.0022-1112.2005.00648.x.
- McKaye, K.R. (1981). "Field observation on death feigning: a unique hunting behavior by the predatory cichlid, Haplochromis livingstoni, of Lake Malawi". Environmental Biology of Fishes. 6 (3–4): 361–365. doi:10.1007/BF00005766.
- Shwartz, Mark (25 January 2007). "Study: Cichlids can determine their social rank by observation". Stanford University. Retrieved 11 December 2018.
- Fernald, Russell D.; Chen, Chun-Chun (25 May 2011). "Visual Information Alone Changes Behavior and Physiology during Social Interactions in a Cichlid Fish (Astatotilapia burtoni)". PLOS One. 6 (5): e20313. Bibcode:2011PLoSO...620313C. doi:10.1371/journal.pone.0020313. ISSN 1932-6203. PMC 3102105. PMID 21633515.
- Knight, Kathryn (1 October 2015). "Fighting cichlids size up opposition with lateral line". Journal of Experimental Biology. 218 (20): 3161. doi:10.1242/jeb.132563. ISSN 1477-9145.
- Fernald, Russell D. (1 January 2017). "Cognitive skills and the evolution of social systems". Journal of Experimental Biology. 220 (1): 103–113. doi:10.1242/jeb.142430. ISSN 1477-9145. PMC 5278620. PMID 28057833.
- Fernald, Russell D.; Kent, Kai R.; Hilliard, Austin T.; Becker, Lisa; Alcazar, Rosa M. (15 August 2016). "Two types of dominant male cichlid fish: behavioral and hormonal characteristics". Biology Open. 5 (8): 1061–1071. doi:10.1242/bio.017640. ISSN 2046-6390. PMC 5004607. PMID 27432479.
- Arnott, Gareth; Ashton, Charlotte; Elwood, Robert W. (23 October 2011). "Lateralization of lateral displays in convict cichlids". Biology Letters. 7 (5): 683–685. doi:10.1098/rsbl.2011.0328. ISSN 1744-9561. PMC 3169077. PMID 21508024.
- Martin, E.; M. Taborsky (1997). "Alternative male mating acttics in a cichlid, Pelvicachromis pulcher: a comparison of reproductive effort and success". Behavioral Ecology and Sociobiology. 41 (5): 311–319. doi:10.1007/s002650050391.
- Egger, Bernd; Klaefiger, Yuri; Theis, Anya; Salzburger, Walter (18 October 2011). "A Sensory Bias Has Triggered the Evolution of Egg-Spots in Cichlid Fishes". PLOS One. 6 (10): e25601. Bibcode:2011PLoSO...625601E. doi:10.1371/journal.pone.0025601. ISSN 1932-6203. PMC 3196499. PMID 22028784.
- Santos, M. Emília; Braasch, Ingo; Boileau, Nicolas; Meyer, Britta S.; Sauteur, Loïc; Böhne, Astrid; Belting, Heinz-Georg; Affolter, Markus; Salzburger, Walter (9 October 2014). "The evolution of cichlid fish egg-spots is linked with a cis-regulatory change". Nature Communications. 5: 5149. Bibcode:2014NatCo...5.5149S. doi:10.1038/ncomms6149. ISSN 2041-1723. PMC 4208096. PMID 25296686.
- Salzburger, Walter; Mack, Tanja; Verheyen, Erik; Meyer, Axel (1 January 2005). "Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes". BMC Evolutionary Biology. 5: 17. doi:10.1186/1471-2148-5-17. ISSN 1471-2148. PMC 554777. PMID 15723698.
- Alonso, F. et al. 2011. Social and reproductive physiology and behavior of the Neotropical cichlid fish Cichlasoma dimerus under laboratory conditions. SciELO, Neotropical Ichthyology, 9(3)
- Nelson, C. M. 1995. Male size, spawning pit size and female mate choice in a lekking cichlid fish. Animal Behavior, 50(6):1587–1599.
- Duponchelle, F. et al. 2008. Parallel life history evolution in mouthbrooding cichlids from the African Great Lakes. Proceedings of the National Academy of Sciences of the United States of America, 105(40):15475-15480.
- Brown, D.H. and Marshall, J. A. 1978. Reproductive Behaviour of the Rainbow Cichlid, Herotilapia multispinosa (Pisces, Cichlidae). Behaviour, 67(¾):299–321.
- Muschik, M. et al. 2012. Convergent evolution within an adaptive radiation of cichlid fishes. Current Biology, 24(18): 2362–2368.
- Wisenden, B. D. et al. 1995. Fin digging and leaf lifting by the convict cichlid, Cichlasoma nigrofasciatum: examples of parental food provisioning. Animal Behaviour, 49(3): 623–631.
- Ota, K. and Kohda, M. 2014. Maternal Food Provisioning in a Substrate-Brooding African Cichlid. Public Library of Science, 9(6):e99094.
- Duponchelle, F. et al. 2008. Parallel life history evolution in mouthbrooding cichlids from the African Great Lakes. Proceedings of the National Academy of Sciences of the United States of America, 105(40):15475-15480.
- Nelson, C. M. 1995. Male size, spawning pit size and female mate choice in a lekking cichlid fish. Animal Behavior, 50(6):1587–1599.
- Sefc, K. M. 2011. Mating and Parental Care in Lake Tanganyika’s Cichlids. International Journal of Evolutionary Biology, 2011: 470875.
- York, R. A. et al. 2015. Evolution of bower building in Lake Malawi cichlid fish: phylogeny, morphology, and behavior. Frontiers in Ecology and Evolution.
- Zardoya, R. et al. 1996. Evolutionary conservation of microsatellite flanking regions and their use in resolving the phylogeny of cichlid fishes (Pisces: Perciformes). Proceedings of the Royal Society of London. Series B: Biological Sciences, 263(1376), 1589–1598.
- Goodwin, N. B. et al. 1998. Evolutionary transitions in parental care in cichlid fish. Proceedings of the Royal Society B, 265(1412): 2265–2272.
- Hulsey, C. D. et al. 2010. Temporal diversification of Central American cichlids. BMC evolutionary biology, 10(1), 279.
- Zardoya, R. et al. 1996. Evolutionary conservation of microsatellite flanking regions and their use in resolving the phylogeny of cichlid fishes (Pisces: Perciformes). Proceedings of the Royal Society of London. Series B: Biological Sciences, 263(1376), 1589–1598.
- Burchard, J. F. 1964. Family structure in the Dwarf Cichlid Apistogramma trifasciatum Eigenmann and Kennedy. Zeitschrift für Tierpsychologie, 22(2): 150–162.
- Dierkes, P. et al. 1999. Reproductive parasitism of broodcare helpers in a cooperatively breeding fish. Behavioral Ecology, 10(5): 510–515.
- Zworykin, D. D. 2001. Parental brood provisioning in cichlid fishes by means of stirring up the bottom substrate: a brief review. Cichlid Research: State of the Art, 9:269–286.
- McKaye, K.R.; N.M. McKaye (1977). "Communal Care and Kidnapping of Young by Parental Cichlids". Evolution. 31 (3): 674–681. doi:10.2307/2407533. JSTOR 2407533. PMID 28563477.
- Ward, J.A.; R.L. Wyman (1977). "Ethology and ecology of cichlid fishes of the genus Etroplus in Sri Lanka: preliminary findings". Environmental Biology of Fishes. 2 (2): 137–145. doi:10.1007/BF00005369.
- Ribbink, A.J.; A.C. Marsh, and B.A. Marsh (1981). "Nest-building and communal care of young by Tilapia rendalli dumeril (pisces, cichlidae) in Lake Malawi". Environmental Biology of Fishes. 6 (2): 219–222. doi:10.1007/BF00002787.
- Steeves, Greg. Neolamprologus brichardi. africancichlids.net. Retrieved 8 April 2008
- Riehl, Rüdiger. Editor.; Baensch, HA (1996). Aquarium Atlas. Germany: Tetra Press. ISBN 978-3-88244-050-8.
- Balon, E.K. (1975). "Reproductive guilds of fishes: a proposal. and definition". Journal of the Fisheries Research Board of Canada. 32 (6): 821–864. doi:10.1139/f75-110.
- Keenleyside, M. H. A. (1991). "Parental Care". Cichlid Fishes: behaviour, ecology and evolution. London: Chapman and Hall. pp. 191–208. ISBN 978-0-412-32200-6.
- Kishida, M.; J.L. Specker (2000). "Paternal Mouthbrooding in the Black-Chinned Tilapia, Sarotherodon melanotheron (Pisces: Cichlidae): Changes in Gonadal Steroids and Potential for Vitellogenin Transfer to Larvae". Hormones and Behavior. 37 (1): 40–48. doi:10.1006/hbeh.1999.1556. PMID 10712857.
- Coleman, R. (January 1999). "Mysterious mouthbrooders". Cichlid News: 32–33.
- Meier, Joana I.; Marques, David A.; Mwaiko, Salome; Wagner, Catherine E.; Excoffier, Laurent; Seehausen, Ole (10 February 2017). "Ancient hybridization fuels rapid cichlid fish adaptive radiations". Nature Communications. 8: 14363. Bibcode:2017NatCo...814363M. doi:10.1038/ncomms14363. PMC 5309898. PMID 28186104.
- Albertson, R. C.; Markert, J. A.; Danley, P. D.; Kocher, T. D. (1999). "Phylogeny of a rapidly evolving clade: The cichlid fishes of Lake Malawi, East Africa". PNAS. 96 (9): 5107–5110. Bibcode:1999PNAS...96.5107A. doi:10.1073/pnas.96.9.5107. PMC 21824. PMID 10220426.
- Muschick, M.; Barluenga, M.; Salzburger, W.; Meyer, A. (2011). "Adaptive phenotypic plasticity in the Midas cichlid fish pharyngeal jaw and its relevance in adaptive radiation". BMC Evolutionary Biology. 11: 116. doi:10.1186/1471-2148-11-116. PMC 3103464. PMID 21529367.
- Barluenga, M.; Meyer, A.; Muschick, M.; Salzburger, W.; Stolting, K. N. (2006). "Sympatric speciation in Nicaraguan crater lake cichlid fish" (PDF). Nature. 439 (7077): 719–23. Bibcode:2006Natur.439..719B. doi:10.1038/nature04325. PMID 16467837.
- IUCN 2010. IUCN Red List of Threatened Species. Version 2010.4. Retrieved 26 April 2011.
- Witte, F., de Zeeuw, M.P. & Brooks, E. (2010). "Haplochromis thereuterion". IUCN Red List of Threatened Species. 2010: e.T185857A8492470. doi:10.2305/IUCN.UK.2010-3.RLTS.T185857A8492470.en.CS1 maint: multiple names: authors list (link)
- Barlow, G. W. (2000). The Cichlid Fishes. Cambridge, MA: Perseus Publishing. ISBN 978-0-7382-0376-8.
- DeWeerdt, S. (28 February 2004). Dark secret of the lake. New Scientist. Retrieved 28 March 2017.
- Lowe-McConnell, R (2009). "Fisheries and cichlid evolution in the African Great Lakes: progress and problems". Freshwater Reviews. 2 (2): 131–151. doi:10.1608/frj-2.2.2.
- rijssel, Van; Witte (2013). "Adaptive responses in resurgent Lake Victoria cichlids over the past 30 years". Evol. Ecol. 27 (2): 253–267. doi:10.1007/s10682-012-9596-9.
- Fiedler, P.L.; and P.M. Kareiva, editors (1998). Conservation Biology: For the Coming Decade. 2nd edition. Pp. 209—210. ISBN 978-0412096617
- Goldschmidt, Witte; Wanink (1993). "Cascading Effects of the Introduced Nile Perch on the Detritivorous/Phytoplanktivorous Species in the Sublittoral Areas of Lake Victoria". Conservation Biology. 7 (3): 686–700. doi:10.1046/j.1523-1739.1993.07030686.x.
- IUCN Red Lists: Geographic Patterns. Eastern Africa. Retrieved 25 March 2017.
- Chapman; Chapman; Chandler (1996). "Wetland ecotones as refugia for endangered fishes". Biological Conservation. 78 (3): 263–270. CiteSeerX 10.1.1.689.2625. doi:10.1016/s0006-3207(96)00030-4.
- McGee; Borstein; Neches; Buescher; Seehausen; Wainwright (2015). "A pharyngeal jaw evolutionary innovation facilitated extinction in Lake Victoria cichlids". Science. 350 (6264): 1077–1079. Bibcode:2015Sci...350.1077M. doi:10.1126/science.aab0800. PMID 26612951.
- De Silva, S.S; Subasinghe, R.P.; Bartley, D.M.; Lowther, A. Tilapias as Alien Aquatics in Asia and the Pacific: A Review. FAO Fisheries Technical Paper. No. 453, 2004.
- Florida Fish and Wildlife Conservation Commission. "Fact Exotic Freshwater Fishes". Retrieved 18 March 2007.
- Sands D (1994) A fishkeepers guide to Central American cichlids. Tetra Press. Belgium pg 59-60.
- Mills D (1993) Aquarium Fish Harper Collins ISBN 0-7322-5012-9
- Konings A (1997) Back to nature guide to Malawi Cichlids Druckhaus Beltz, Germany. p. 13-23
- Leibel WS (1993) A fishkeepers guide to South American cichlids. Tetra Press. Belgium pg 12–14.
- Smith P.F.; Konings A.; Kornfield I. (July 2003). "Hybrid origin of a cichlid population in Lake Malawi: implications for genetic variation and species diversity". Molecular Ecology. 12 (9): 2497–2504. doi:10.1046/j.1365-294X.2003.01905.x. PMID 12919487.
- Wood, A. B., and Jordan, D. R.: Fertility of roach × bream hybrids, Rutilus rutilus (L.) × Abramis brama (L.), and their identification. Journal of Fish Biology 30, pp 249–261, 1987
- Matt Clarke. "Frequently asked questions on Parrot cichlids". Practical Fishkeeping. Archived from the original on 26 September 2007. Retrieved 20 October 2006.
- "It's The Frankenstein Monster of the Fish World: The Blood Parrot!". AquaFriend.com. 27 October 2002. Archived from the original on 16 May 2006. Retrieved 5 December 2006.
- Arnold W (2003) Singapore's 'lucky' pet Luohan can outnumber people in homes. International Herald Tribune. 1 July.
- Crayfish the latest fad among pet lovers New Straits Times (Malaysia) (2004) 3 September
- Flower Horn: Joy in homes, a pest in rivers. New Straits Times (Malaysia) (2004) 14 July.
- Norton, J (1982). "Angelfish genetics". Freshwater and Marine Aquarium Magazine. 5: 4.
- Koh, TL; Khoo, G; Fan, LQ; Phang, VPE (1999). "Genetic diversity among wild forms and cultivated varieties of Discus (Symphysodon spp.) as revealed by Random Amplified Polymorphic DNA (RAPD) fingerprinting". Aquaculture. 173 (1–4): 485–497. doi:10.1016/s0044-8486(98)00478-5.
- Kornfield I (1991) Genetics. In: Cichlid Fishes: behaviour, ecology and evolution Ed. Keenleyside MHA. Chapman and Hall, London. p. 109-115.
- Itzkovich, J; Rothbard, S; Hulata, G (1981). "Inheritance of pink body colouration in Cichlasoma nigrofasciatum Günther (Pisces, Cichlidae)". Genetica. 55: 15–16. doi:10.1007/bf00133997.
- McAndrew, CJ; Roubal, FR; Roberts, RJ; Bullock, AM; McEwan, IM (1988). "The genetics and history of red, blond, and associated color variants in Oreochromis niloticus". Genetica. 76 (2): 127–137. doi:10.1007/bf00058811.
- Linke H, Staeck L (1994) American cichlids I: Dwarf Cichlids. A handbook for their identification, care and breeding. Tetra Press. Germany. ISBN 1-56465-168-1
- Norton J (1994) Notched – An Angelfish Deformity Freshwater And Marine Aquarium magazine 17:(3)
- Dunz, A.R.; Schliewen, U.K. (2013). "Molecular phylogeny and revised classification of the haplotilapiine cichlid fishes formerly referred to as "Tilapia"". Molecular Phylogenetics and Evolution. 68 (1): 64–80. doi:10.1016/j.ympev.2013.03.015. PMID 23542002.
- De la Maza-Benignos, M., Ornelas-García, C. P., Lozano-Vilano, M.d.L., García-Ramírez, M.E. & Doadrio, I. (2015). "Phylogeographic analysis of genus Herichthys (Perciformes: Cichlidae), with descriptions of Nosferatu new genus and H. tepehua n. sp". Hydrobiologia. 748 (1): 201–231. doi:10.1007/s10750-014-1891-8. hdl:10261/126238.CS1 maint: multiple names: authors list (link)
Further reading
- Barlow, G. W. (2000). The Cichlid fishes. Cambridge MA: Perseus Publishing.
- "Cichlidae". Integrated Taxonomic Information System.: National Museum of Natural History, Washington, D.C., 2004-05-11).
- Sany, R. H. (2012). Taxonomy of Cichlids and Angel. (Web publication).
External links
| Wikimedia Commons has media related to Cichlidae. |
- Cichlid at Curlie
- The Cichlid Fishes of Lake Malawi by Dr. Michael Oliver.
- Vision in Cichlids: Ecomorphology of vision in haplochromine cichlids of Lake Victoria by Dr. H.J. van der Meer.
- . Encyclopædia Britannica. 6 (11th ed.). 1911. p. 360.