Lek mating
A lek is an aggregation of male animals gathered to engage in competitive displays and courtship rituals, known as lekking, to entice visiting females which are surveying prospective partners to mate with.[1] A lek can also indicate an available plot of space able to be utilized by displaying males to defend their own share of territory and/or mates. Leks are commonly formed before or during the breeding season. A lekking species is characterised by male displays, strong female mate choice, and the conferring of indirect benefits to males. Although most prevalent among birds such as black grouse, lekking is also found in a wide range of vertebrates including some bony fish, amphibians, reptiles, and mammals, and arthropods including crustaceans and insects.
.jpg)
A classical lek consists of male territories in visual and auditory range of each other. An exploded lek, as seen in the kakapo (the owl parrot), has more widely separated territories, but still in auditory range.
Lekking is associated with an apparent paradox: strong sexual selection by females for specific male traits ought to erode genetic diversity by Fisherian runaway, but diversity is maintained and runaway does not occur. Many attempts have been made to explain it away,[2][3][4][5] but the paradox remains.
Etymology
The term derives from the Swedish lek, a noun which typically denotes pleasurable and less rule-bound games and activities ("play", as by children). English use of lek dates to the 1860s. Llewelyn Lloyd's The Game birds and wild fowl of Sweden and Norway (1867) introduces it (capitalised and in single quotes, as 'Lek') explicitly as a Swedish term.[6]
Taxonomic range
Lekking was originally described in the Tetraonidae (grouse, boldface in cladogram), in particular the black grouse (Swedish: "orrlek") and capercaillie (Swedish: "tjäderlek"), but it is widely distributed phylogenetically among other birds, and in many other animal groups within the vertebrates and the arthropods, as shown in the cladogram.[1][7][8] The presence of a group name means that some species in that group lek; groups with no lekking members are not shown.
| Nephrozoa |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Lekking behaviour

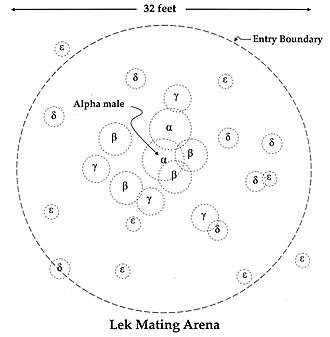
There are two types of lekking arrangement: classical and exploded. In the classic lekking system, male territories are in visual and auditory range of their neighbours. In an exploded lek, males are further away from one another than they would be in a classic lek. Males in an exploded lek are outside of visual range of one another, but they stay within earshot.[35] Exploded lek territories are much larger than classic systems and more variable in size.[36] A well-known example of exploded leks is the "booming" call of the kakapo, the males of which position themselves many kilometres apart from one another to signal to potential mates.[37]
Lek territories of different taxa are stable and do not vary in terms of size and location.[38] Males often return to the same mating sites because of female fidelity.[39] Avian females such as the black grouse and great snipe are faithful to males and not to mating sites.[40] Successful males congregate in the same area as the previous breeding season because it is familiar to them, while females return to reunite with their males. Females do not return to a mating site if their male partner is not present.[40] Another possible explanation for lek stability is from male hierarchies within a lek. In some species of manakins, subordinate betas may inherit an alpha's display site, increasing the chances of female visitation.[40] Rank may also contribute to the stability of lek size, as lower ranking males may congregate to achieve a perceived optimal size to attract females.[41]
Some species of ant, such as red harvester ant,[26] as well as certain bee species, like Tetragonisca angustula and Trigona spinipes exhibit lek-like mating patterns. Males form reproductive aggregations, congregating and collectively give off a pheromone that attracts reproductive females in proportion to the number of males present.[42][43]
Lek mating is relatively common among paper wasps. For example, Polistes dominula males often fight with other males in mid-air to demonstrate their superiority and attractiveness. Males that lose fly away from the lek. Females fly through leks or perch near lekking areas to observe males before making choices on mates; they use the highly conspicuous abdominal spots on males, which are highly variable in size and shape, to aid in mate choice. Males with smaller, more elliptically shaped spots are more dominant over other males and are preferred by females to males with larger, more irregularly-shaped spots.[44] In comparison, Mischocyttarus flavitarsis males choose a perch site near female hibernation areas, rub their abdomens to mark their territory and wait 6–7 weeks for a female to approach. If an intruder approaches, the owner of the site lunges and grapples the other wasp. Typically, they fall off the perch site and finish the fight on the ground.[45]
Costs and benefits
The main benefit for both sexes is mating success. For males, the costs stem from females' preferences. The traits that are selected for may be energetically costly to maintain and may cause increased predation. For example, increased vocalization rate caused a decrease in the mass of male great snipes.[46] Other costs can derive from male combat. For example, male great snipes regularly fight to display dominance or defend their territory, with females preferring victorious males.[46] Aggressive male black grouse are preferred over non-aggressive males and when the males fight they tear feathers from each other's tails.[47] Lekking is associated with sexual dimorphism across a range of bird taxa.[7]
At first glance, it may seem that females receive no direct benefits from lekking, since the males are only contributing genes to the offspring in the absence of parental care or other benefits.[48] However, lekking reduces the cost of female searching because the congregating of males makes mate selection easier.[49] Females do not have to travel as far, since they are able to evaluate and compare multiple males within the same vicinity. Further, having the males in one place may reduce the amount of time a female is vulnerable to predators. When under predatory pressure, female marbled reed frogs have been seen consistently to choose leks near their release sites, and high male calling rates reduced female search time.[50]
Female mating preferences
A meta analysis of 27 species found that qualities such as lekking size, male display rate, and the rate of male aggression exhibit positive correlation with male success rates.[1] A positive correlation was also found between attendance, magnitude of exaggerated traits, age, frequency of fights, and mating success.[1] This female preference leads to mating skew, with some males being more successful at copulating with females. The variation in mating success is quite large in lek mating systems with 70–80% of matings being attributed to only 10–20% of the males present.[51]
The lek paradox

Since sexual selection by females for specific male trait values should erode genetic diversity, the maintenance of genetic variation in lekking species constitutes a paradox in evolutionary biology. Many attempts have been made to explain it away, but the paradox remains.[52] There are two conditions in which the lek paradox arises. The first is that males contribute only genes and the second is that female preference does not affect fecundity.[53] Female choice should lead to directional runaway selection, resulting in a greater prevalence for the selected traits. Stronger selection should lead to impaired survival, as it decreases genetic variance and ensures that more offspring have similar traits.[54] However, lekking species do not exhibit runaway selection. In a lekking reproductive system, what male sexual characteristics can signal to females is limited, as the males provide no resources to females or parental care to their offspring.[55] This implies that a female gains indirect benefits from her choice in the form of "good genes" for her offspring.[56]
Amotz Zahavi argued that male sexual characteristics only convey useful information to the females if these traits confer a handicap on the male.[57][58] Zahavi's handicap principle may offer a resolution to the lek paradox, for if females select for the condition of male ornaments, then their offspring have better fitness. Another potential resolution to the lek paradox is Rowe and Houle's theory that sexually selected traits depend on physical condition, which might in turn, summarize many genetic loci.[56] This is the genic capture hypothesis, which describes how a significant amount of the genome is involved in shaping the traits that are sexually selected.[55] There are two assumptions in the genic capture hypothesis: the first is that sexually selected traits are dependent upon condition, and the second is that general condition is attributable to high genetic variance.[56] In addition, W. D. Hamilton and M. Zuk proposed that sexually selected traits might signal resistance to parasites.[59] One resolution to the lek paradox involves female preferences and how preference alone does not cause a drastic enough directional selection to diminish the genetic variance in fitness.[60] Another conclusion is that the preferred trait is not naturally selected for or against and the trait is maintained because it implies increased attractiveness to the male.[53]

Evolution
Several possible mechanisms have been proposed as to why males cluster into leks, including the hotshot, hotspot, black hole, kin selection, and predation protection hypotheses, as described below.
Hotshot hypothesis
The hotshot hypothesis is the only model that attributes males as the driving force behind aggregation. The hotshot model hypothesizes that attractive males, known as hotshots, garner both female and male attention.[2] Females go to the hotshots because they are attracted to these males. Other males form leks around these hotshots as a way to lure females away from the hotshot. A manipulative experiment using the little bustard, Tetrax tetrax, was done to test the various lek evolution models.[4] The experiment involved varying the size and sex ratio of leks using decoys. To test whether or not the presence of a hotshot determined lek formation, a hotshot little bustard decoy was placed within a lek. After the fake hotshot was added to the lek, both male and female visitation to the lek increased, tending to confirm the hypothesis.[4]
Hotspot model

The hotspot model considers the female density to be the catalyst for the clustering of males. This model predicts that leks will form where females tend to reside as a way to increase female interaction.[3] Female manakin traffic has been observed to be concentrated around leks, bathing sites, and fruiting areas, with males aggregated near the most visited fruiting resources.[3] The hotspot model also predicts that lek size is dependent upon the number of females inhabiting a patch of land.[2] To test if the number of females affects lek formation, a group of female little bustard decoys were added to a lek. The presence of these female decoys did not have an effect on lek size, tending to refute the hypothesis.[4]
Blackhole model
The blackhole model proposes that females have a preference for neither size nor type of male, but rather that females tend to be mobile and mate wherever leks may be located.[4] This model predicts that female mobility is a response to male harassment.[61] This prediction is difficult to test, but there was a negative correlation found between male aggressiveness and female visitation in the little bustard population.[4] Evidence supporting the black hole model is mainly found in ungulates.[39]
Kin selection
An alternative hypothesis for lekking is kin selection, which assumes that males within a lek are related to one another. As females rarely mate outside of leks, it is advantageous for males to form leks.[5] Although not all males within a lek mate with a female, the unmated males still receive fitness benefits. Kin selection explains that related males congregate to form leks, as a way to attract females and increase inclusive fitness.[40] In some species, the males at the leks show a high degree of relatedness, but this does not apply as a rule to lek-forming species in general.[62][63][64] In a few species such as peacocks and black grouse, leks are composed of brothers and half-brothers. The lower-ranking males gain some fitness benefit by passing their genes on through attracting mates for their brothers, since larger leks attract more females. Peacocks recognize and lek with their brothers, even if they have never met before.[65]
Predation protection
Another hypothesis is predation protection, or the idea that there is a reduction in individual predation risk in a larger group.[4] This could work both for the males in within the group as well as any female who visits the lek.[66] Protection also explains the presence of mixed leks, when a male of one species joins a lek of another species for protection from a common set of predators. This occurs with manakins,[67] as well as other birds such as grouse species.[68]
References
- Fiske, P.; Rintamaki, P. T.; Karvonen, E. (1998). "Mating success in lekking males: a meta-analysis". Behavioral Ecology. 9 (4): 328–338. doi:10.1093/beheco/9.4.328.
- Foster, M. S.; Beehler, B. M. (1998). "Hotshots, Hotspots, and Female Preferences in the Organization of Lek Mating Systems". The American Naturalist. 131 (2): 203–219. doi:10.1086/284786.
- Théry, M. (1992). "The evolution of leks through female choice: Differential clustering and space utilization in six sympatric manakins". Behavioral Ecology and Sociobiology. 30 (3–4): 227–237. doi:10.1007/bf00166707.
- Jiguet, F.; Bretagnolle, V. (2006). "Manipulating lek size and composition using decoys: An experimental investigation of lek evolution models". The American Naturalist. 168 (6): 758–768. doi:10.1086/508808. PMID 17109318.
- Durães, R.; Loiselle, B. A.; Blake, J. G. (2008). "Spatial and temporal dynamics at manakin leks: Reconciling lek traditionality with male turnover". Behavioral Ecology and Sociobiology. 62 (12): 1947–1957. doi:10.1007/s00265-008-0626-0.
- Lloyd, Llewelyn (1867). The Game Birds and Wild Fowl of Sweden and Norway. London: Frederick Warne & Co. pp. 219ff.. Lloyd also loans 'Lek-ställe' (Swedish lekställe, "play-place") for "pairing ground".
- Oakes, E. J. (1992). "Lekking and the Evolution of Sexual Dimorphism in Birds: Comparative Approaches". The American Naturalist. 140 (4): 665–684. doi:10.1086/285434. ISSN 0003-0147.
- Bourke, Andrew F. G. (2014). "Hamilton's rule and the causes of social evolution". Philosophical Transactions of the Royal Society B: Biological Sciences. The Royal Society. 369 (1642): 20130362. doi:10.1098/rstb.2013.0362. ISSN 0962-8436.
- Ponomarenko, I. Ja (1965). "Comparative characteristics of some biological indices of the bottom stages of 0-group cod belonging to the 1956, 1958, 1959, 1960 and 1961 year-classes". Spec. Publ. Int. Comm. Northwest Atlantic Fish: 349–354.
- Loiselle, Paul V. (December 1982). "Male Spawning-Partner Preference in an Arena-Breeding Teleost Cyprinodon macularius californiensis Girard (Atherinomorpha: Cyprinodontidae)". The American Naturalist. 120 (6): 721–732. doi:10.1086/284026.
- Nelson, C. M. 1995. Male size, spawning pit size and female mate choice in a lekking cichlid fish. Animal Behavior, 50(6):1587–1599.
- Emlen, Stephen T (1976). "Lek Organization and Mating Strategies in the Bullfrog". Behavioral Ecology and Sociobiology. 1 (3): 283–313. doi:10.1007/bf00300069.
- Knopp, T.; Heimovirta, M.; Kokko, H.; Merila, J. (2008). "Do Male Moor Frogs (Rana arvalis) Lek with Kin?". Molecular Ecology. 17 (10): 2522–530. doi:10.1111/j.1365-294x.2008.03748.x. PMID 18422930.
- Vitousek, Maren N.; Mitchell, Mark A.; Woakes, Anthony J.; Niemack, Michael D.; Wikelski, Martin; Tregenza, Tom (2007). "High Costs of Female Choice in a Lekking Lizard". PLOS ONE. 2 (6): e567. Bibcode:2007PLoSO...2..567V. doi:10.1371/journal.pone.0000567. PMC 1891434. PMID 17593966.

- Toth, C. A.; Parsons, S. (2013). "Is lek breeding rare in bats?". Journal of Zoology. 291 (1): 3–11. doi:10.1111/jzo.12069.
- Bradbury, J. W. (1977). "Lek Mating Behavior in the Hammer-headed Bat". Zeitschrift für Tierpsychologie. 45 (3): 225–255. doi:10.1111/j.1439-0310.1977.tb02120.x.
- Bro-Jørgensen, Jakob; Durant, Sarah M. (2003). "Mating strategies of topi bulls: getting in the centre of attention" (PDF). Animal Behaviour. 65 (3): 585–594. doi:10.1006/anbe.2003.2077.
- Clutton-Brock, Tim H.; et al. (1988). "Passing the buck: resource defence, lek breeding and mate choice in fallow deer". Behavioral Ecology and Sociobiology. 23 (5): 281–296. doi:10.1007/BF00300575. JSTOR 4600219.
- Apollonio, Marco; Festa-Bianchet, Marco; Mari, Franco (1989). "Correlates of copulatory success in a fallow deer lek". Behavioral Ecology and Sociobiology. 25 (2): 89–97. doi:10.1007/BF00302925. JSTOR 4600315.
- Buechner, Helmut K.; Roth, H. Daniel (1974). "The lek system in Uganda kob antelope" (PDF). American Zoologist. 14 (1): 145–162. doi:10.1093/icb/14.1.145.
- Soto, Karim H.; Trites, Andrew W. (2011). "South American sea lions in Peru have a lek‐like mating system" (PDF). Marine Mammal Science. 27 (2): 306–333. doi:10.1111/j.1748-7692.2010.00405.x.
- Boness, Daryl J.; et al. (2006). "Mating tactics and mating system of an aquatic-mating pinniped: the harbor seal, Phoca vitulina". Behavioral Ecology and Sociobiology. 61 (1): 119–130. CiteSeerX 10.1.1.571.550. doi:10.1007/s00265-006-0242-9.
- Croll, George A.; McClintock, James B. (2000). "An evaluation of lekking behavior in the fiddler crab Uca spp". Journal of Experimental Marine Biology and Ecology. 254 (1): 109–121. doi:10.1016/s0022-0981(00)00276-8. ISSN 0022-0981.
- Cappa, F.; Bruschini, C.; Cervo, R.; Turillazzi, S.; Beani, L. (2013). "Males do not like the working class: male sexual preference and recognition of functional castes in a primitively eusocial wasp". Animal Behaviour. 86 (4): 801–810. doi:10.1016/j.anbehav.2013.07.020.
- Alcock, John (1983). "Consistency in the Relative Attractiveness of a Set of Landmark Territorial Sites to Two Generations of Male Tarantula Hawk Wasps (Hymenoptera: Pompilidae)". Animal Behaviour. 31: 74–80. doi:10.1016/s0003-3472(83)80174-2.
- Davidson, Diane W. (1982). "Sexual Selection in Harvester Ants". Behavioral Ecology and Sociobiology. 10 (4): 245–50. doi:10.1007/bf00302813.
- Kimsey, Lynn Siri (1980). "The behaviour of male orchid bees (Apidae, Hymenoptera, Insecta) and the question of leks". Animal Behaviour. 28 (4): 996–1004. doi:10.1016/S0003-3472(80)80088-1.
- Lederhouse, Robert C. (1982). "Territorial Defense and Lek Behavior of the Black Swallowtail Butterfly, Papilio polyxenes". Behavioral Ecology and Sociobiology. 10 (2): 109–118. doi:10.1007/bf00300170. JSTOR 4599468.
- Mallet, James (1984). "Sex roles in the ghost moth Hepialus humuli (L.) and a review of mating in the Hepialidae(Lepidoptera)". Zoological Journal of the Linnean Society. 79: 67–82.
- Turner, J. R. G. (2015). "The flexible lek: Phymatopus hecta the gold swift demonstrates the evolution of lekking and male swarming via a hotspot (Lepidoptera: Hepialidae". Biological Journal of the Linnean Society. 114: 184–201.
- Parsons, P. A. (1977). "Lek Behavior in Drosophila (Hirtodrosophila) Polypori Malloch-An Australian Rainforest Species". Evolution. 31 (1): 223–225. doi:10.2307/2407561. ISSN 0014-3820. JSTOR 2407561.
- Marshall, S. A. (2000). "Agonistic behaviour and generic synonymy in Australian Clusiidae (Diptera)". Studia Dipterologica. 7: 3–9.
- Starr, Cecie; Taggart, Ralph (1992). Biology: The Unity and Diversity of Life (6th ed.). Wadsworth. ISBN 978-0-534-16566-6.
- Hall, Edward T. (1966). The Hidden Dimension. Anchor Books. ISBN 978-0-385-08476-5.
- Trail, P. W. (1990). "Why Should Lek-Breeders be Monomorphic?". Evolution. 44 (7): 1837–1852. doi:10.2307/2409512. JSTOR 2409512. PMID 28567800.
- Jiguet, F.; Arroyo, B.; Bretagnolle, V. (2000). "Lek mating systems: a case study in the Little Bustard Tetrax tetrax". Behavioural Processes. 51 (1–3): 63–82. doi:10.1016/s0376-6357(00)00119-4. PMID 11074312.
- Merton, Don V.; Morris, Rodney B.; Atkinson, Ian A. E. (1984). "Lek behaviour in a parrot: The Kakapo Strigops habroptilus of New Zealand". Ibis. 126 (3): 277–283. doi:10.1111/j.1474-919X.1984.tb00250.x.
- Durães, R.; Loiselle; Parker, P. G.; Blake, J. G. (2009). "Female mate choice across spatial scales: influence of lek and male attributes on mating success of blue-crowned manakins". Proceedings of the Royal Society B: Biological Sciences. 276 (1663): 1875–1881. doi:10.1098/rspb.2008.1752. PMC 2674486. PMID 19324796.
- Isvaran, K (2005). "Variation in male mating behaviour within ungulate populations: patterns and processes" (PDF). Current Science. 89 (7): 1192–1199.
- Duval, E. H. (2013). "Female mate fidelity in a lek mating system and its implications for the evolution of cooperative lekking behaviour". The American Naturalist. 181 (2): 213–22. doi:10.1086/668830. PMID 23348775.
- Hernandez, M. L.; Houston, A. I.; Mcnamara, J. M. (1999). "Male rank and optimal lek size". Behavioral Ecology. 10: 73–79. doi:10.1093/beheco/10.1.73.
- Velthuis, Hayo H.W.; Koedam, Dirk; Imperatriz-Fonseca, Vera L (2005). "The males of Melipona and other stingless bees, and their mothers". Apidologie. 36 (2): 169–185. doi:10.1051/apido:2005014.
- Prato, M; Soares, A E E (July 2013). "Production of Sexuals and Mating Frequency in the Stingless Bee Tetragonisca angustula (Latreille) (Hymenoptera, Apidae)". Neotropical Entomology. 42 (5): 474–482. doi:10.1007/s13744-013-0154-0. PMID 23949986.
- Izzo, Amanda; Elizabeth, A.; Tibbetts, E. (2012). "Spotting the Top Male: Sexually Selected Signals in Male Polistes dominulus Wasps". Animal Behaviour. 83 (3): 839–845. doi:10.1016/j.anbehav.2012.01.005.
- Litte, Marcia (1979). "Mischocyttarus flavitarsis in Arizona: Social and Nesting Biology of a Polistine Wasp". Zeitschrift für Tierpsychologie. 50 (3): 282–312. doi:10.1111/j.1439-0310.1979.tb01033.x.
- Höglund, J.; Kalais, J. A.; Fiske, P. (1992). "The costs of secondary sexual characters in the lekking great snipe (Gallinago media)". Behavioral Ecology and Sociobiology. 30 (5): 309–315. doi:10.1007/bf00170596.
- Alatalo, R. V; Höglund, J.; Lundberg, A. (1991). "Lekking in the black grouse: a test of male viability". Nature. 352 (6331): 155–156. Bibcode:1991Natur.352..155A. doi:10.1038/352155a0.
- Reynolds, J. D.; Gross, M. R. (1990). "Costs and Benefits of Female Mate Choice: Is There a Lek Paradox?". The American Naturalist. 136 (2): 230–243. doi:10.1086/285093. ISSN 0003-0147.
- Wickman, P.; Jansson, P. (1997). "An estimate of female mate searching costs in the lekking butterfly Coenonympha pamphilus". Behavioral Ecology and Sociobiology. 40 (5): 321–328. doi:10.1007/s002650050348.
- Grafe, T. Ulmar (May 1997). "Costs and benefits of mate choice in the lek-breeding reed frog, Hyperolius marmoratus". Animal Behaviour. 53 (5): 1103–1117. doi:10.1006/anbe.1996.0427.
- Mackenzie, A.; Reynolds, J. D.; Sutherland, W. (1995). "Variation in Male Mating Success on Leks". The American Naturalist. 145 (4): 633–652. doi:10.1086/285759.
- Miller, Christine; Moore, Allen (2007). "A potential resolution to the lek paradox through indirect genetic effects". Proceedings of the Royal Society B: Biological Sciences. 274 (1615): 1279–1286. doi:10.1098/rspb.2007.0054. PMC 2176171. PMID 17341455.
- Kirkpatrick, M. (1982). "Sexual Selection and the Evolution of Female Choice". Evolution. 36 (1): 1–12. doi:10.2307/2407961. JSTOR 2407961. PMID 28581098.
- Kirkpatrick, M.; Ryan, M. (1991). "The evolution of mating preferences and the paradox of the lek". Nature. 350 (6313): 33–38. Bibcode:1991Natur.350...33K. doi:10.1038/350033a0.
- Tomkins, Joseph L. (June 2004). "Genic capture and resolving the lek paradox" (PDF). Trends in Ecology & Evolution. 19 (6). Archived from the original (PDF) on 21 March 2006.
- Rowe, Locke; Houle, David (1996). "The lek paradox and the capture of genetic variance by condition dependent traits". Proceedings of the Royal Society B: Biological Sciences. 263 (1375): 1415–1421. Bibcode:1996RSPSB.263.1415R. doi:10.1098/rspb.1996.0207. JSTOR 50503.
- Zahavi, Amotz (1975). "Mate selection—a selection for a handicap" (PDF). Journal of Theoretical Biology. 53 (1): 205–214. CiteSeerX 10.1.1.586.3819. doi:10.1016/0022-5193(75)90111-3. PMID 1195756.
- Iwasa, Y.; Pomiankowski, A.; Nee, S. (1991). "The Evolution of Costly Mate Preferences II: The 'Handicap' Principle". Evolution. 45 (6): 1431–1442. doi:10.2307/2409890. JSTOR 2409890. PMID 28563835.
- Hamilton, W. D.; Zuk, M. (1982). "Heritable true fitness and bright birds: A role for parasites?" (PDF). Science. 218 (4570): 384–387. Bibcode:1982Sci...218..384H. doi:10.1126/science.7123238. PMID 7123238.
- Pomiankowski, A; Moller, A. P. (1995). "A Resolution of the Lek Paradox". Proceedings of the Royal Society B: Biological Sciences. 260 (1357): 21–29. doi:10.1098/rspb.1995.0054.
- Clutton-Brock, T. H.; Price, O. F.; Maccou, A. D. C. (1991). "Mate retention, harassment, and the evolution of ungulate leks". Behavioral Ecology. 3 (3): 234–242. doi:10.1093/beheco/3.3.234.
- Loiselle, B. A.; Ryder, Thomas B.; Durães, Renata; Tori, Wendy; Blake, John G.; Parker, Patricia G. (2007). "Kin Selection Does Not Explain Male Aggregation at Leks of 4 Manakin Species". Behavioral Ecology. 18 (2): 287–291. doi:10.1093/beheco/arl081.
- McDonald, D. B.; Potts, W. K. (1994). "Cooperative display and relatedness among males in a lek-mating bird". Science. 266 (5187): 1030–1032. Bibcode:1994Sci...266.1030M. doi:10.1126/science.7973654. PMID 7973654.
- Hoglund, J. (2003). "Lek-kin in birds – Provoking theory and surprising new results" (PDF). Annales Zoologici Fennici. 40: 249–253.
- Petrie, Marion; Krupa, Andrew; Terry, Burke (1999). "Peacocks lek with relatives even in the absence of social and environmental cues". Nature. 401 (6749): 155–157. Bibcode:1999Natur.401..155P. doi:10.1038/43651.
- Concannon, Moira R.; Stein, Adam C.; Uy, J. Albert C. (2012). "Kin selection may contribute to lek evolution and trait introgression across an avian hybrid zone". Molecular Ecology. 21 (6): 1477–1486. doi:10.1111/j.1365-294X.2012.05474.x. ISSN 1365-294X. PMID 22320709.
- Brumfield, Robb T.; Liu, Liang; Lum, David E.; Edwards, Scott V. (1 October 2008). "Comparison of Species Tree Methods for Reconstructing the Phylogeny of Bearded Manakins (Aves: Pipridae, Manacus) from Multilocus Sequence Data". Systematic Biology. 57 (5): 719–731. doi:10.1080/10635150802422290. ISSN 1063-5157. PMID 18853359.
- Gibson, Robert M.; Aspbury, Andrea S.; McDaniel, Leonard L. (2002). "Active formation of mixed–species grouse leks: a role for predation in lek evolution?". Proceedings of the Royal Society of London B: Biological Sciences. 269 (1509): 2503–2507. doi:10.1098/rspb.2002.2187. ISSN 0962-8452. PMC 1691199. PMID 12573063.