Beak
The beak, bill, and/or rostrum is an external anatomical structure found mostly in birds, but also in non-avian dinosaurs and some mammals. A beak is used for eating and for preening, manipulating objects, killing prey, fighting, probing for food, courtship and feeding young. The terms beak and rostrum are also used to refer to a similar mouth part in some ornithischians, pterosaurs, turtles, cetaceans, dicynodonts, anuran tadpoles, monotremes (i.e. echidnas and platypuses, which have a beak-like structure), sirens, pufferfish, billfishes and cephalopods.
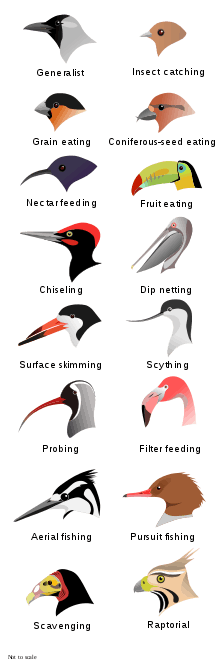
Although beaks vary significantly in size, shape, color and texture, they share a similar underlying structure. Two bony projections—the upper and lower mandibles—are covered with a thin keratinized layer of epidermis known as the rhamphotheca. In most species, two holes known as nares lead to the respiratory system.
Etymology
Although the word beak was, in the past, generally restricted to the sharpened bills of birds of prey,[1] in modern ornithology, the terms beak and bill are generally considered to be synonymous.[2] The word, which dates from the 13th century, comes from the Middle English bec, which itself comes from the Latin beccus.[3]
Anatomy
.jpg)
Although beaks vary significantly in size and shape from species to species, their underlying structures have a similar pattern. All beaks are composed of two jaws, generally known as the upper mandible (or maxilla) and lower mandible (or mandible).[4] The upper, and in some cases the lower, mandibles are strengthened internally by a complex three-dimensional network of bony spicules (or trabeculae) seated in soft connective tissue and surrounded by the hard outer layers of the beak.[5][6] The avian jaw apparatus is made up of two units: one four-bar linkage mechanism and one five-bar linkage mechanism.[7]
Mandibles
The upper mandible is supported by a three-pronged bone called the intermaxillary. The upper prong of this bone is embedded into the forehead, while the two lower prongs attach to the sides of the skull. At the base of the upper mandible a thin sheet of nasal bones is attached to the skull at the nasofrontal hinge, which gives mobility to the upper mandible, allowing it to move upwards and downwards.[2]
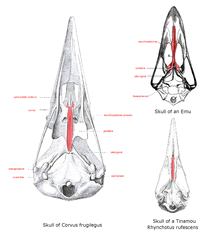
The base of the upper mandible, or the roof when seen from the mouth, is the palate, the structure of which differs greatly in the ratites. Here, the vomer is large and connects with premaxillae and maxillopalatine bones in a condition termed as a "paleognathous palate". All other extant birds have a narrow forked vomer that does not connect with other bones and is then termed as neognathous. The shape of these bones varies across the bird families.
The lower mandible is supported by a bone known as the inferior maxillary bone—a compound bone composed of two distinct ossified pieces. These ossified plates (or rami), which can be U-shaped or V-shaped,[4] join distally (the exact location of the joint depends on the species) but are separated proximally, attaching on either side of the head to the quadrate bone. The jaw muscles, which allow the bird to close its beak, attach to the proximal end of the lower mandible and to the bird's skull.[8] The muscles that depress the lower mandible are usually weak, except in a few birds such as the starlings and the extinct Huia, which have well-developed digastric muscles that aid in foraging by prying or gaping actions.[9] In most birds, these muscles are relatively small as compared to the jaw muscles of similarly sized mammals.[10]
Rhamphotheca
The outer surface of the beak consists of a thin horny sheath of keratin called the rhamphotheca,[2][8] which can be subdivided into the rhinotheca of the upper mandible and the gnathotheca of the lower mandible.[11] This covering arises from the Malpighian layer of the bird's epidermis,[11] growing from plates at the base of each mandible.[12] There is a vascular layer between the rhamphotheca and the deeper layers of the dermis, which is attached directly to the periosteum of the bones of the beak.[13] The rhamphotheca grows continuously in most birds, and in some species, the color varies seasonally.[14] In some alcids, such as the puffins, parts of the rhamphotheca are shed each year after the breeding season, while some pelicans shed a part of the bill called a "bill horn" that develops in the breeding season.[15][16][17]
While most extant birds have a single seamless rhamphotheca, species in a few families, including the albatrosses[11] and the emu, have compound rhamphothecae that consist of several pieces separated and defined by softer keratinous grooves.[18] Studies have shown that this was the primitive ancestral state of the rhamphotheca, and that the modern simple rhamphotheca resulted from the gradual loss of the defining grooves through evolution.[19]
Tomia

The tomia (singular tomium) are the cutting edges of the two mandibles.[20] In most birds, these range from rounded to slightly sharp, but some species have evolved structural modifications that allow them to handle their typical food sources better.[21] Granivorous (seed-eating) birds, for example, have ridges in their tomia, which help the bird to slice through a seed's outer hull.[22] Most falcons have a sharp projection along the upper mandible, with a corresponding notch on the lower mandible. They use this "tooth" to sever their prey's vertebrae fatally or to rip insects apart. Some kites, principally those that prey on insects or lizards, also have one or more of these sharp projections,[23] as do the shrikes.[24] Some fish-eating species, e.g., the mergansers, have sawtooth serrations along their tomia, which help them to keep hold of their slippery, wriggling prey.[25]
Birds in roughly 30 families have tomia lined with tight bunches of very short bristles along their entire length. Most of these species are either insectivores (preferring hard-shelled prey) or snail eaters, and the brush-like projections may help to increase the coefficient of friction between the mandibles, thereby improving the bird's ability to hold hard prey items.[26] Serrations on hummingbird bills, found in 23% of all hummingbird genera, may perform a similar function, allowing the birds to effectively hold insect prey. They may also allow shorter-billed hummingbirds to function as nectar thieves, as they can more effectively hold and cut through long or waxy flower corollas.[27] In some cases, the color of a bird's tomia can help to distinguish between similar species. The snow goose, for example, has a reddish-pink bill with black tomia, while the whole beak of the similar Ross's goose is pinkish-red, without darker tomia.[28]
Culmen

The culmen is the dorsal ridge of the upper mandible.[29] Likened by ornithologist Elliott Coues to the ridge line of a roof, it is the "highest middle lengthwise line of the bill" and runs from the point where the upper mandible emerges from the forehead's feathers to its tip.[30] The bill's length along the culmen is one of the regular measurements made during bird banding (ringing)[31] and is particularly useful in feeding studies.[32] There are several standard measurements that can be made—from the beak's tip to the point where feathering starts on the forehead, from the tip to the anterior edge of the nostrils, from the tip to the base of the skull, or from the tip to the cere (for raptors and owls)[33]—and scientists from various parts of the world generally favor one method over another.[32] In all cases, these are chord measurements (measured in a straight line from point to point, ignoring any curve in the culmen) taken with calipers.[31]
The shape or color of the culmen can also help with the identification of birds in the field. For example, the culmen of the parrot crossbill is strongly decurved, while that of the very similar red crossbill is more moderately curved.[34] The culmen of a juvenile common loon is all dark, while that of the very similarly plumaged juvenile yellow-billed loon is pale towards the tip.[35]
Gonys
The gonys is the ventral ridge of the lower mandible, created by the junction of the bone's two rami, or lateral plates.[36] The proximal end of that junction—where the two plates separate—is known as the gonydeal angle or gonydeal expansion. In some gull species, the plates expand slightly at that point, creating a noticeable bulge; the size and shape of the gonydeal angle can be useful in identifying between otherwise similar species. Adults of many species of large gulls have a reddish or orangish gonydeal spot near the gonydeal expansion.[37] This spot triggers begging behavior in gull chicks. The chick pecks at the spot on its parent's bill, which in turn stimulates the parent to regurgitate food.[38]
Commissure
Depending on its usage, commissure may refer to the junction of the upper and lower mandibles,[39] or alternately, to the full-length apposition of the closed mandibles, from the corners of the mouth to the tip of the beak.[40]
Gape

In bird anatomy, the gape is the interior of the open mouth of a bird, and the gape flange is the region where the two mandibles join together at the base of the beak.[41] The width of the gape can be a factor in the choice of food.[42]

Gapes of juvenile altricial birds are often brightly coloured, sometimes with contrasting spots or other patterns, and these are believed to be an indication of their health, fitness and competitive ability. Based on this, the parents decide how to distribute food among the chicks in the nest.[43] Some species, especially in the families Viduidae and Estrildidae, have bright spots on the gape known as gape tubercles or gape papillae. These nodular spots are conspicuous even in low light.[44] A study examining the nestling gapes of eight passerine species found that the gapes were conspicuous in the ultraviolet spectrum (visible to birds but not to humans).[45] Parents may, however, not rely solely on the gape coloration, and other factors influencing their decision remain unknown.[46]
Red gape color has been shown in several experiments to induce feeding. An experiment in manipulating brood size and immune system with barn swallow nestlings showed the vividness of the gape was positively correlated with T-cell–mediated immunocompetence, and that larger brood size and injection with an antigen led to a less vivid gape.[47] Conversely, the red gape of the common cuckoo (Cuculus canorus) did not induce extra feeding in host parents.[48] Some brood parasites, such as the Hodgson's hawk-cuckoo (C. fugax), have colored patches on the wing that mimic the gape color of the parasitized species.[49]
When born, the chick's gape flanges are fleshy. As it grows into a fledgling, the gape flanges remain somewhat swollen and can thus be used to recognize that a particular bird is young.[50] By the time it reaches adulthood, the gape flanges will no longer be visible.
Nares
Most species of birds have external nares (nostrils) located somewhere on their beak. The nares are two holes—circular, oval or slit-like in shape—which lead to the nasal cavities within the bird's skull, and thus to the rest of the respiratory system.[51] In most bird species, the nares are located in the basal third of the upper mandible. Kiwis are a notable exception; their nares are located at the tip of their bills.[21] A handful of species have no external nares. Cormorants and darters have primitive external nares as nestlings, but these close soon after the birds fledge; adults of these species (and gannets and boobies of all ages, which also lack external nostrils) breathe through their mouths.[11] There is typically a septum made of bone or cartilage that separates the two nares, but in some families (including gulls, cranes and New World vultures), the septum is missing.[11] While the nares are uncovered in most species, they are covered with feathers in a few groups of birds, including grouse and ptarmigans, crows, and some woodpeckers.[51] The feathers over a ptarmigan's nostrils help to warm the air it inhales,[52] while those over a woodpecker's nares help to keep wood particles from clogging its nasal passages.[53]
Species in the bird order Procellariformes have nostrils enclosed in double tubes which sit atop or along the sides of the upper mandible.[51] These species, which include the albatrosses, petrels, diving petrels, storm petrels, fulmars and shearwaters, are widely known as "tubenoses".[54] A number of species, including the falcons, have a small bony tubercule which projects from their nares. The function of this tubercule is unknown. Some scientists suggest it may act as a baffle, slowing down or diffusing airflow into the nares (and thus allowing the bird to continue breathing without damaging its respiratory system) during high-speed dives, but this theory has not been proved experimentally. Not all species that fly at high speeds have such tubercules, while some species which fly at low speeds do.[55]
Operculum

The nares of some birds are covered by an operculum (plural opercula), a membraneous, horny or cartilaginous flap.[56][57] In diving birds, the operculum keeps water out of the nasal cavity;[56] when the birds dive, the impact force of the water closes the operculum.[58] Some species which feed on flowers have opercula to help to keep pollen from clogging their nasal passages,[56] while the opercula of the two species of Attagis seedsnipe help to keep dust out.[59] The nares of nestling tawny frogmouths are covered with large dome-shaped opercula, which help to reduce the rapid evaporation of water vapor, and may also help to increase condensation within the nostrils themselves—both critical functions, since the nestlings get fluids only from the food their parents bring them. These opercula shrink as the birds age, disappearing completely by the time they reach adulthood.[60] In pigeons, the operculum has evolved into a soft swollen mass that sits at the base of the bill, above the nares;[61] though it is sometimes referred to as the cere, this is a different structure.[62] Tapaculos are the only birds able to move their opercula.[51]
Rosette
Some species, such as the puffin, have a fleshy rosette, sometimes called a "gape rosette",[63] at the corners of the beak. In the puffin, this is grown as part of its display plumage.[64]
Cere
Birds from a handful of families—including raptors, owls, skuas, parrots, turkeys and curassows—have a waxy structure called a cere (from the Latin cera, which means "wax") or ceroma[65][66] which covers the base of their bill. This structure typically contains the nares, except in the owls, where the nares are distal to the cere. Although it is sometimes feathered in parrots,[67] the cere is typically bare and often brightly colored.[21] In raptors, the cere is a sexual signal which indicates the "quality" of a bird; the orangeness of a Montagu's harrier's cere, for example, correlates to its body mass and physical condition.[68] The cere color of young Eurasian scops-owls has an ultraviolet (UV) component, with a UV peak that correlates to the bird's mass. A chick with a lower body mass has a UV peak at a higher wavelength than a chick with a higher body mass does. Studies have shown that parent owls preferentially feed chicks with ceres that show higher wavelength UV peaks, that is, lighter-weight chicks.[69]
The color or appearance of the cere can be used to distinguish between males and females in some species. For example, the male great curassow has a yellow cere, which the female (and young males) lack.[70] The male budgerigar's cere is blue, while the female's is pinkish or brown.[71]
Nail

All birds of the family Anatidae (ducks, geese, and swans) have a nail, a plate of hard horny tissue at the tip of the beak.[72] This shield-shaped structure, which sometimes spans the entire width of the beak, is often bent at the tip to form a hook.[73] It serves different purposes depending on the bird's primary food source. Most species use their nails to dig seeds out of mud or vegetation,[74] while diving ducks use theirs to pry molluscs from rocks.[75] There is evidence that the nail may help a bird to grasp things; species which use strong grasping motions to secure their food (such as when catching and holding onto a large squirming frog) have very wide nails.[76] Certain types of mechanoreceptors, nerve cells that are sensitive to pressure, vibration or touch, are located under the nail.[77]
The shape or color of the nail can sometimes be used to help distinguish between similar-looking species or between various ages of waterfowl. For example, the greater scaup has a wider black nail than does the very similar lesser scaup.[78] Juvenile "grey geese" have dark nails, while most adults have pale nails.[79] The nail gave the wildfowl family one of its former names: "Unguirostres" comes from the Latin ungus, meaning "nail" and rostrum, meaning "beak".[73]
Rictal bristles
Rictal bristles are stiff hair-like feathers that arise around the base of the beak.[80] They are common among insectivorous birds, but are also found in some non-insectivorous species.[81] Their function is uncertain, although several possibilities have been proposed.[80] They may function as a "net", helping in the capture of flying prey, although to date, there has been no empirical evidence to support this idea.[82] There is some experimental evidence to suggest that they may prevent particles from striking the eyes if, for example, a prey item is missed or broken apart on contact.[81] They may also help to protect the eyes from particles encountered in flight, or from casual contact from vegetation.[82] There is also evidence that the rictal bristles of some species may function tactilely, in a manner similar to that of mammalian whiskers (vibrissae). Studies have shown that Herbst corpuscles, mechanoreceptors sensitive to pressure and vibration, are found in association with rictal bristles. They may help with prey detection, with navigation in darkened nest cavities, with the gathering of information during flight or with prey handling.[82]
Egg tooth

Full-term chicks of most bird species have a small sharp, calcified projection on their beak, which they use to chip their way out of their egg.[83] Commonly known as an egg tooth, this white spike is generally near the tip of the upper mandible, though some species have one near the tip of their lower mandible instead, and a few species have one on each mandible.[84] Despite its name, the projection is not an actual tooth, as the similarly-named projections of some reptiles are; instead, it is part of the integumentary system, as are claws and scales.[85] The hatching chick first uses its egg tooth to break the membrane around an air chamber at the wide end of the egg. Then it pecks at the eggshell while turning slowly within the egg, eventually (over a period of hours or days) creating a series of small circular fractures in the shell.[86] Once it has breached the egg's surface, the chick continues to chip at it until it has made a large hole. The weakened egg eventually shatters under the pressure of the bird's movements.[87] The egg tooth is so critical to a successful escape from the egg that chicks of most species will perish unhatched if they fail to develop one.[84] However, there are a few species which do not have egg teeth. Megapode chicks have an egg tooth while still in the egg but lose it before hatching,[86] while kiwi chicks never develop one; chicks of both families escape their eggs by kicking their way out.[88] Most chicks lose their egg teeth within a few days of hatching,[83] though petrels keep theirs for nearly three weeks[87] and marbled murrelets have theirs for up to a month.[89] Generally, the egg tooth drops off, though in songbirds it is reabsorbed.[87]
Color
The color of a bird's beak results from concentrations of pigments—primarily melanins and carotenoids—in the epidermal layers, including the rhamphotheca.[90] Eumelanin, which is found in the bare parts of many bird species, is responsible for all shades of gray and black; the denser the deposits of pigment found in the epidermis, the darker the resulting color. Phaeomelanin produces "earth tones" ranging from gold and rufous to various shades of brown.[91] Although it is thought to occur in combination with eumelanin in beaks which are buff, tan, or horn-colored, researchers have yet to isolate phaeomelanin from any beak structure.[92] More than a dozen types of carotenoids are responsible for the coloration of most red, orange, and birds never have yellow beaks.[93] The hue of the color is determined by the precise mix of red and yellow pigments, while the saturation is determined by the density of the deposited pigments. For example, bright red is created by dense deposits of mostly red pigments, while dull yellow is created by diffuse deposits of mostly yellow pigments. Bright orange is created by dense deposits of both red and yellow pigments, in roughly equal concentrations.[94] Beak coloration helps to make displays using those beaks more obvious.[95]
Birds are capable of seeing colors in the ultraviolet range, and some species are known to have ultraviolet peaks of reflectance (indicating the presence of ultraviolet color) on their beaks.[96] The presence and intensity of these peaks may indicate a bird's fitness,[68] sexual maturity or pair bond status.[96] King and emperor penguins, for example, show spots of ultraviolet reflectance only as adults. These spots are brighter on paired birds than on courting birds. The position of such spots on the beak may be important in allowing birds to identify conspecifics. For instance, the very similarly-plumaged king and emperor penguins have UV-reflective spots in different positions on their beaks.[96]
In general, beak color depends on a combination of the bird's hormonal state and diet. Colors are typically brightest as the breeding season approaches, and palest after breeding.[37]
Dimorphism

The size and shape of the beak can vary across species as well as between them; in some species, the size and proportions of the beak vary between males and females. This allows the sexes to utilize different ecological niches, thereby reducing intraspecific competition.[97] For example, females of nearly all shorebirds have longer bills than males of the same species,[98] and female American avocets have beaks which are slightly more upturned than those of males.[99] Males of the larger gull species have bigger, stouter beaks than those of females of the same species, and immatures can have smaller, more slender beaks than those of adults.[100] Many hornbills show sexual dimorphism in the size and shape of both beaks and casques, and the female huia's slim, decurved bill was nearly twice as long as the male's straight, thicker one.[101]
Color can also differ between sexes or ages within a species. Typically, such a color difference is due to the presence of androgens. For example, in house sparrows, melanins are produced only in the presence of testosterone; castrated male house sparrows—like female house sparrows—have brown beaks. Castration also prevents the normal seasonal color change in the beaks of male black-headed gulls and indigo buntings.[102]
Functions

Birds may bite or stab with their beaks to defend themselves.[103] Some species use their beaks in displays of various sorts. As part of his courtship, for example, the male garganey touches his beak to the blue speculum feathers on his wings in a fake preening display, and the male Mandarin duck does the same with his orange sail feathers.[104] A number of species use a gaping, open beak in their fear and/or threat displays. Some augment the display by hissing or breathing heavily, while others clap their beak. The platypus uses its bill to navigate underwater, detect food, and dig. The bill contains electroreceptors and mechanoreceptors, causing muscular contractions to help detect prey. It is one of the few species of mammals to use electroreception.[105][106]
Preening
The beak of birds plays a role in removing skin parasites (ectoparasites) such as lice. It is mainly the tip of the beak that does this. Studies have shown that inserting a bit to stop birds from using the tip results in increased parasite loads in pigeons.[107] Birds that have naturally deformed beaks have also been noted to have higher levels of parasites.[108][109][110][111] It is thought that the overhang at the end of the top portion of the beak (that is the portion that begins to curve downwards) slides against the lower beak to crush parasites.[107]
This overhang of the beak is thought to be under stabilising natural selection. Very long beaks are thought to be selected against because they are prone to a higher number of breaks, as has been demonstrated in rock pigeons.[112] Beaks with no overhang would be unable to effectively remove and kill ectoparasites as mentioned above. Studies have supported there is a selection pressure for an intermediate amount of overhang. Western Scrub Jays who had more symmetrical bills (i.e. those with less of an overhang), were found to have higher amounts of lice when tested.[113] The same pattern has been seen in surveys of Peruvian birds.[114]
Additionally, because of the role beaks play in preening, this is evidence for coevolution of the beak overhang morphology and body morphology of parasites. Artificially removing the ability to preen in birds, followed by readdition of preening ability was shown to result in changes in body size in lice. Once the ability of the birds to preen was reintroduced, the lice were found to show declines in body size suggesting they may evolve in response to preening pressures from birds[107] who could respond in turn with changes in beak morphology.
Communication
A number of species, including storks, some owls, frogmouths and the noisy miner, use bill clapping as a form[107] of communication.[115]
Heat exchange
Studies have shown that some birds use their beaks to rid themselves of excess heat. The toco toucan, which has the largest beak relative to the size of its body of any bird species, is capable of modifying the blood flow to its beak. This process allows the beak to work as a "transient thermal radiator", reportedly rivaling an elephant's ears in its ability to radiate body heat.[116] Measurements of the bill sizes of several species of American sparrows found in salt marshes along the North American coastlines show a strong correlation with summer temperatures recorded in the locations where the sparrows breed; latitude alone showed a much weaker correlation. By dumping excess heat through their bills, the sparrows are able to avoid the water loss which would be required by evaporative cooling—an important benefit in a windy habitat where freshwater is scarce.[117] Several ratites, including the common ostrich, the emu and the southern cassowary, use various bare parts of their bodies (including their beaks) to dissipate as much as 40% of their metabolic heat production.[118] Alternately, studies have shown that birds from colder climates (higher altitudes or latitudes and lower environmental temperatures) have smaller beaks, lessening heat loss from that structure.[119]
Billing

During courtship, mated pairs of many bird species touch or clasp each other's bills. Termed billing (also nebbing in British English),[120] this behavior appears to strengthen pair bonding.[121] The amount of contact involved varies among species. Some gently touch only a part of their partner's beak while others clash their beaks vigorously together.[122]
Gannets raise their bills high and repeatedly clatter them, the male puffin nibbles at the female's beak, the male waxwing puts his bill in the female's mouth and ravens hold each other's beaks in a prolonged "kiss".[123] Billing can also be used as a gesture of appeasement or subordination. Subordinate Canada jay routinely bill more dominant birds, lowering their body and quivering their wings in the manner of a young bird food begging as they do so.[124] A number of parasites, including rhinonyssids and Trichomonas gallinae are known to be transferred between birds during episodes of billing.[125][126]
Usage of the term has spread beyond avian behavior; "billing and cooing" in reference to human courtship (particularly kissing) has been in use since Shakespeare's time,[127] and derives from the courtship of doves.[128]
Beak trimming
Because the beak is a sensitive organ with many sensory receptors, beak trimming (sometimes referred to as 'debeaking') is "acutely painful"[129] to the birds it is performed on. It is nonetheless routinely done to intensively farmed poultry flocks, particularly laying and broiler breeder flocks, because it helps reduce the damage the flocks inflict on themselves due to a number of stress-induced behaviors, including cannibalism, vent pecking and feather pecking. A cauterizing blade or infrared beam is used to cut off about half of the upper beak and about a third of the lower beak. Pain and sensitivity can persist for weeks or months after the procedure, and neuromas can form along the cut edges. Food intake typically decreases for some period after the beak is trimmed. However, studies show that trimmed poultry's adrenal glands weigh less, and their plasma corticosterone levels are lower than those found in untrimmed poultry, indicating that they are less stressed overall.[129]
A similar but separate practice, usually performed by an avian veterinarian or an experienced birdkeeper, involves clipping, filing or sanding the beaks of captive birds for health purposes – in order to correct or temporarily alleviate overgrowths or deformities and better allow the bird to go about its normal feeding and preening activities.[130] Amongst raptor keepers, this practice is commonly known as "coping".[131]
Bill tip organ

The bill tip organ is a region found near the tip of the bill in several types of birds that forage particularly by probing. The region has a high density of nerve endings known as the corpuscles of Herbst. This consists of pits in the bill surface which in the living bird is occupied by cells that sense pressure changes. The assumption is that this allows the bird to perform 'remote touch', which means that it can detect movements of animals which the bird does not directly touch. Bird species known to have a 'bill-tip organ' includes members of ibisis, shorebirds of the family Scolopacidae, and kiwis.[132]
There is a suggestion that across these species, the bill tip organ is more well developed among species foraging in wet habitats (water column or soft mud) than in species using a more terrestrial foraging. However, it has been described in terrestrial birds too, including parrots, who are known for their dextrous extractive foraging techniques. Unlike probing foragers, the tactile pits in parrots are embedded in the hard keratin (or rhamphotheca) of the bill, rather than the bone, and along the inner edges of the curved bill, rather than being on the outside of the bill.[133]
See also
References
- Partington, Charles Frederick (1835). The British cyclopæedia of natural history: combining a scientific classification of animals, plants, and minerals. Orr & Smith. p. 417.
- Proctor and Lynch (1998), p. 66.
- "Beak". Merriam-Webster. Retrieved 1 July 2016.
- Coues (1890), p. 147.
- Gill (1995), p. 149.
- Seki, Yasuaki; Bodde, Sara G; Meyers, Marc A; Meyers (2009). "Toucan and hornbill beaks: A comparative study" (PDF). Acta Biomaterialia. 6 (2): 331–343. doi:10.1016/j.actbio.2009.08.026. PMID 19699818. Archived from the original (PDF) on 2012-04-02.
- Beyond the Beak: Modeling avian cranial kinesis and the evolution of bird skull shapes
- Gill (1995), p. 148.
- Mayr, Gerald (2005). "A new eocene Chascacocolius-like mousebird (Aves: Coliiformes) with a remarkable gaping adaptation" (PDF). Organisms, Diversity & Evolution. 5 (3): 167–171. doi:10.1016/j.ode.2004.10.013.
- Kaiser, Gary W. (2007). The Inner Bird: Anatomy and Evolution. Vancouver, BC: UBC Press. p. 19. ISBN 978-0-7748-1343-3.
- Campbell and Lack (1995), p. 47.
- Girling (2003), p. 4.
- Samour (2000), p. 296.
- Bonser RH & Mark S Witter (1993). "Indentation hardness of the bill keratin of the European Starling" (PDF). The Condor. 95 (3): 736–738. doi:10.2307/1369622. JSTOR 1369622.
- Beddard, Frank E. (1898). The structure and classification of birds. London: Longmans, Green and Co. p. 5.
- Pitocchelli, Jay; John F. Piatt; Harry R. Carter (2003). "Variation in plumage, molt, and morphology of the Whiskered Auklet (Aethia pygmaea) in Alaska". Journal of Field Ornithology. 74 (1): 90–98. doi:10.1648/0273-8570-74.1.90.
- Knopf, F. L. (1974). "Schedule of presupplemental molt of white pelicans with notes on the bill horn" (PDF). Condor. 77 (3): 356–359. doi:10.2307/1366249. JSTOR 1366249.
- Chernova, O. F.; Fadeeva, E. O. (2009). "The peculiar architectonics of contour feathers of the emu (Dromaius novaehollandiae, Struthioniformes)". Doklady Biological Sciences. 425: 175–179. doi:10.1134/S0012496609020264.
- Hieronymus, Tobin L.; Witmer, Lawrence M. (2010). "Homology and Evolution of Avian Compound Rhamphothecae". The Auk. 127 (3): 590–604. doi:10.1525/auk.2010.09122.
- Campbell and Lack (1985), p. 598.
- Stettenheim, Peter R. (2000). "The Integumentary Morphology of Modern Birds—An Overview". Integrative and Comparative Biology. 40 (4): 461–477. doi:10.1093/icb/40.4.461. Archived from the original (PDF) on 2012-04-20.
- Klasing, Kirk C. (1999). "Avian gastrointestinal anatomy and physiology". Seminars in Avian and Exotic Pet Medicine. 8 (2): 42–50. doi:10.1016/S1055-937X(99)80036-X.
- Ferguson-Lees, James; Christie, David A. (2001-01-01). Raptors of the World. London: Christopher Helm. p. 66. ISBN 978-0-7136-8026-3.
- Harris, Tony; Franklin, Kim (2000). Shrikes and Bush-Shrikes. London: Christopher Helm. p. 15. ISBN 978-0-7136-3861-5.
- Campbell and Lack (1985), p. 48.
- Gosner, Kenneth L. (June 1993). "Scopate Tomia: An Adaptation for Handling Hard-shelled Prey?" (PDF). The Wilson Bulletin. 105 (2): 316–324.
- Ornelas, Juan Francisco. "Serrate Tomia: An Adaptation for Nectar Robbing in Hummingbirds?" (PDF). The Auk. 111 (3): 703–710.
- Madge, Steve; Burn, Hilary (1988). Wildfowl. London: Christopher Helm. pp. 143–144. ISBN 978-0-7470-2201-5.
- Campbell and Lack (1995), p. 127.
- Coues (1890), p. 152.
- Pyle, Peter; Howell, Steve N. G.; Yunick, Robert P.; DeSante, David F. (1987). Identification Guide to North America Passerines. Bolinas, CA: Slate Creek Press. pp. 6–7. ISBN 978-0-9618940-0-9.
- Borras, A.; Pascual, J.; Senar, J. C. (Autumn 2000). "What Do Different Bill Measures Measure and What Is the Best Method to Use in Granivorous Birds?" (PDF). Journal of Field Ornithology. 71 (4): 606–611. doi:10.1648/0273-8570-71.4.606. JSTOR 4514529.
- Campbell and Lack (1995), p. 342.
- Mullarney, Svensson, Zetterström and Grant (1999), p. 357.
- Mullarney, Svensson, Zetterström and Grant (1999), p. 15.
- Campbell and Lack (1985), p. 254.
- Howell (2007), p. 23.
- Russell, Peter J.; Wolfe, Stephen L.; Hertz, Paul E.; Starr, Cecie (2008). Biology: The Dynamic Science. Vol. 2. Belmont, CA: Thomson Brooks/Cole. p. 1255. ISBN 978-0-495-01033-3.
- Coues (1890), p. 155.
- Campbell & Lack (1985), p. 105.
- Newman, Kenneth B. (2000). Newman's birds by colour. Struik. p. 14. ISBN 978-1-86872-448-2.
- Wheelwright, NT (1985). "Fruit size, gape width and the diets of fruit-eating birds" (PDF). Ecology. 66 (3): 808–818. doi:10.2307/1940542. JSTOR 1940542. Archived from the original (PDF) on 2016-04-08. Retrieved 2013-10-31.
- Soler, J. J.; Avilés, J. M. (2010). Halsey, Lewis George (ed.). "Sibling Competition and Conspicuousness of Nestling Gapes in Altricial Birds: A Comparative Study". PLoS ONE. 5 (5): e10509. doi:10.1371/journal.pone.0010509. PMC 2865545. PMID 20463902.
- Hauber, Mark & Rebecca M. Kilner (2007). "Coevolution, communication, and host-chick mimicry in parasitic finches: who mimics whom?" (PDF). Behav. Ecol. Sociobiol. 61 (4): 497–503. doi:10.1007/s00265-006-0291-0. Archived from the original (PDF) on 2012-03-20.
- Sarah Hunt; Rebecca M. Kilner; Naomi E. Langmore; Andrew T. D. Bennett (2003). "Conspicuous, ultravioletrich mouth colours in begging chicks". Biology Letters. 270: S25–8. doi:10.1098/rsbl.2003.0009. PMC 1698012. PMID 12952627.
- Schuetz, Justin G. (October 2005). "Reduced growth but not survival of chicks with altered gape patterns". Animal Behaviour. 70 (4): 839–848. doi:10.1016/j.anbehav.2005.01.007. ISSN 0003-3472.
- Saino, Nicola; Ambrosini, Roberto; Martinelli, Roberta; Ninni, Paola;; Møller, Anders Pape (2003). "Gape coloration reliably reflects immunocompetence of barn swallow (Hirundo rustica) nestlings" (PDF). Behavioral Ecology. 14 (1): 16–22. doi:10.1093/beheco/14.1.16. Archived from the original (PDF) on 11 July 2011. Retrieved 27 June 2010.CS1 maint: uses authors parameter (link)
- Noble, D. G.; Davies, N.B.; Hartley, I. R.; McRae, S. B. (July 1999). "The Red Gape of the Nestling Cuckoo (Cuculus canorus) Is Not a Supernormal Stimulus for Three Common Hosts". Behaviour. 136 (9): 759–777. doi:10.1163/156853999501559. JSTOR 4535638.
- Tanaka, Keita D.; Morimoto, Gen; Ueda, Keisuke (2005). "Yellow wing-patch of a nestling Horsfield's hawk cuckoo Cuculus fugax induces miscognition by hosts: mimicking a gape?". Journal of Avian Biology. 36 (5): 461–64. doi:10.1111/j.2005.0908-8857.03439.x. Archived from the original on 2012-10-21.
- Zickefoose, Julie. "Backyard Mystery Birds". Bird Watcher's Digest. Retrieved 2010-06-25.
- Campbell and Lack (1985), p. 375.
- Gellhorn, Joyce (2007). White-tailed Ptarmigan: Ghosts of the Alpine Tundra. Boulder, CO: Johnson Books. p. 110. ISBN 978-1-55566-397-1.
- Ehrlich, Paul R.; Dobkin, David S.; Wheye, Darryl (1998). The Birder's Handbook: A Field Guide to the Natural History of North American Birds. New York, NY: Simon and Schuster. p. 209. ISBN 978-0-671-65989-9.
- Carboneras, Carlos (1992). "Family Diomedeidae (Albatrosses)". In del Hoyo, Josep; Elliott, Andrew; Sargatal, Jordi (eds.). Handbook of Birds of the World, Volume 1: Ostrich to Ducks. Barcelona: Lynx Edicions. p. 199. ISBN 978-84-87334-10-8.
- Capainolo, Peter; Butler, Carol (2010). How Fast Can a Falcon Dive?. New Brunswick, NJ: Rutgers University Press. p. 51. ISBN 978-0-8135-4790-9.
- Gill (1995), p. 117.
- Whitney, William Dwight; Smith, Benjamin Eli (1911). The Century Dictionary and Cyclopedia, volume 6. New York: The Century Company. p. 4123. LCCN 11031934.
- Bock, Walter J. (1989). "Organisms as Functional Machines: A Connectivity Explanation". American Zoologist. 29 (3): 1119–1132. doi:10.1093/icb/29.3.1119. JSTOR 3883510.
- Tudge, Colin (2009). The Bird: A Natural History of Who Birds Are, Where They Came From, and How They Live. New York, NY: Crown Publishers. p. 140. ISBN 978-0-307-34204-1.
- Kaplan, Gisela T. (2007). Tawny Frogmouth. Collingwood, Victoria: Csiro Publishing. pp. 40–41. ISBN 978-0-643-09239-6.
- Campbell and Lack (1985), p. 84
- Coues (1898), p. 151.
- Mike P. Harris (2014). "Aging Atlantic Puffins Fratercula arctica in summer and winter" (PDF). Seabird. Centre for Ecology & Hydrology. 27: 22–40. Archived from the original (PDF) on June 11, 2016.
- "Skomer Island Puffin Factsheet" (PDF). www.welshwildlife.org. May 2011.
- Webster's Unabridged Dictionary of the English Language
- Eleanor Lawrence (2008). Henderson's Dictionary of Biology (14th ed.). Pearson Benjamin Cummings Prentice Hall. p. 111. ISBN 978-0-321-50579-8.
- Jupiter, Tony; Parr, Mike (2010). Parrots: A Guide to Parrots of the World. A&C Black. p. 17. ISBN 978-1-4081-3575-4.
- Mougeo, François; Arroyo, Beatriz E. (22 June 2006). "Ultraviolet reflectance by the cere of raptors". Biology Letters. 2 (2): 173–176. doi:10.1098/rsbl.2005.0434. PMC 1618910. PMID 17148356.
- Parejo, Deseada; Avilés, Jesús M.; Rodriguez, Juan (23 April 2010). "Visual cues and parental favouritism in a nocturnal bird". Biology Letters. 6 (2): 171–173. doi:10.1098/rsbl.2009.0769. PMC 2865047. PMID 19864276.
- Leopold, Aldo Starker (1972). Wildlife of Mexico: The Game Birds and Mammals. Berkeley, CA: University of California Press. p. 202. ISBN 978-0-520-00724-6.
- Alderton, David (1996). A Birdkeeper's Guide to Budgies. Tetra Press. p. 12.
- King and McLelland (1985), p. 376.
- Elliot, Daniel Giraud (1898). The Wild Fowl of the United States and British Possessions. New York, NY: F. P. Harper. p. xviii. LCCN 98001121.
- Perrins, Christopher M. (1974). Birds. London, UK: Collins. p. 24. ISBN 978-0-00-212173-6.
- Petrie, Chuck (2006). Why Ducks Do That: 40 Distinctive Duck Behaviors Explained and Photographed. Minocqua, WI: Willow Creek Press. p. 31. ISBN 978-1-59543-050-2.
- Goodman, Donald Charles; Fisher, Harvey I. (1962). Functional Anatomy of the Feeding Apparatus in Waterfowl (Aves:Anatidae). Carbondale, IL: Southern Illinois University Press. p. 179. OCLC 646859135.
- King and McLelland (1985), p. 421.
- Dunn, Jon L.; Alderfer, Jonathan, eds. (2006). Field Guide to the Birds of North America (5 ed.). Washington, DC: National Geographic. p. 40. ISBN 978-0-7922-5314-3.
- Mullarney, Svensson, Zetterström and Grant (1999), p. 40.
- Lederer, Roger J. "The Role of Avian Rictal Bristles" (PDF). The Wilson Bulletin. 84 (2): 193–197.
- Conover, Michael R.; Miller, Don E. (November 1980). "Rictal Bristle Function in Willow Flycatcher" (PDF). The Condor. 82 (4): 469–471. doi:10.2307/1367580. JSTOR 1367580.
- Cunningham, Susan J.; Alley, Maurice R.; Castro, Isabel (January 2011). "Facial Bristle Feather Histology and Morphology in New Zealand Birds: Implications for Function". Journal of Morphology (PDF). 272 (1): 118–128. doi:10.1002/jmor.10908. PMID 21069752.
- Campbell and Lack (1985), p. 178.
- Perrins, Christopher M.; Attenborough, David; Arlott, Norman (1987). New Generation Guide to the Birds of Britain and Europe. Austin, TX: University of Texas Press. p. 205. ISBN 978-0-292-75532-1.
- Clark, Jr., George A. (September 1961). "Occurrence and Timing of Egg Teeth in Birds" (PDF). The Wilson Bulletin. 73 (3): 268–278.
- Gill (1995), p. 427.
- Gill (1995), p. 428.
- Harris, Tim, ed. (2009). National Geographic Complete Birds of the World. Washington, DC: National Geographic. p. 23. ISBN 978-1-4262-0403-6.
- Kaiser, Gary W. (2007). The Inner Bird: Anatomy and Evolution. Vancouver, BC: University of Washington Press. p. 26. ISBN 978-0-7748-1344-0.
- Ralph, Charles L. (May 1969). "The Control of Color in Birds". American Zoologist. 9 (2): 521–530. doi:10.1093/icb/9.2.521. JSTOR 3881820. PMID 5362278.
- Hill (2010), p. 62.
- Hill (2010), p. 63.
- Hill (2010), p. 64.
- Hill (2010), p. 66
- Rogers and Kaplan (2000), p. 155.
- Jouventin, Pierre; Nolan, Paul M.; Örnborg, Jonas; Dobson, F. Stephen (February 2005). "Ultraviolet Spots in King and Emperor Penguins". The Condor. 113 (3): 144–150. doi:10.1650/7512.
- Campbell, Bernard Grant, ed. (1972). Sexual Selection and the Descent of Man: The Darwinian Pivot. New Brunswick, NJ: Transaction Publishers. p. 186. ISBN 978-0-202-02005-1.
- Thompson, Bill; Blom, Eirik A. T.; Gordon, Jeffrey A. (2005). Identify Yourself: The 50 Most Common Birding Identification Challenges. New York: Houghton Mifflin Harcourt. p. 128. ISBN 978-0-618-51469-4.
- O'Brien, Michael; Crossley, Richard; Karlson, Kevin (2006). The Shorebird Guide. New York: Houghton Mifflin. p. 76. ISBN 978-0-618-43294-3.
- Howell (2007), p. 21.
- Campbell and Lack (1995), p. 48.
- Parkes, A. S.; Emmens, C. W. (1944). "Effect of Androgens and Estrogens on Birds". In Harris, Richard S.; Thimann, Kenneth Vivian (eds.). Vitamins and hormones, volume 2. New York, NY: Academic Press. p. 371. ISBN 978-0-12-709802-9.
- Samour (2000), p. 7.
- Rogers and Kaplan (2000), p. 20.
- https://www.reed.edu/biology/professors/srenn/pages/teaching/web_2007/myp_site/
- http://www.livescience.com/27572-platypus.html
- Clayton, null; Lee, null; Tompkins, null; Brodie, null (September 1999). "Reciprocal Natural Selection on Host-Parasite Phenotypes" (PDF). The American Naturalist. 154 (3): 261–270. doi:10.1086/303237. hdl:10536/DRO/DU:30056229. ISSN 1537-5323. PMID 10506542.
- Pomeroy, D.E (February 1962). "Birds with abnormal bills" (PDF). British Birds. 55.
- Boyd (1951). "A survey of parasitism of the Staling Sturnus Vulgaris L. in North America". Journal of Parasitology. 37 (1): 56–84. doi:10.2307/3273522. JSTOR 3273522. PMID 14825028.
- Worth (1940). "A note on the Dissemination of Mallophage". Bird Banding. 11: 23, 24.
- Ash (1960). "A study of the mallophaga of birds with particular reference to their ecology". Ibis. 102: 93–110. doi:10.1111/j.1474-919X.1960.tb05095.x.
- Clayton, Dale H.; Moyer, Brett R.; Bush, Sarah E.; Jones, Tony G.; Gardiner, David W.; Rhodes, Barry B.; Goller, Franz (2005-04-22). "Adaptive significance of avian beak morphology for ectoparasite control". Proceedings of the Royal Society of London B: Biological Sciences. 272 (1565): 811–817. doi:10.1098/rspb.2004.3036. ISSN 0962-8452. PMC 1599863. PMID 15888414.
- Moyer, Brett R.; Peterson, A. Townsend; Clayton, Dale H. (2002). "Influence of bill shape on ectoparasite load in western scrub-jays" (PDF). The Condor. 104 (3): 675–678. doi:10.1650/0010-5422(2002)104[0675:iobsoe]2.0.co;2. hdl:1808/16618. ISSN 0010-5422.
- Clayton, D. H.; Walther, B. A. (2001-09-01). "Influence of host ecology and morphology on the diversity of Neotropical bird lice". Oikos. 94 (3): 455–467. doi:10.1034/j.1600-0706.2001.940308.x. ISSN 1600-0706.
- Rogers and Kaplan (2000), p. 83.
- Tattersall, Glenn J.; Andrade, Denis V.; Abe, Augusto S. (24 July 2009). "Heat Exchange from the Toucan Bill Reveals a Controllable Vascular Thermal Radiator". Science. 325 (5949): 468–470. doi:10.1126/science.1175553. PMID 19628866.
- Greenbert, Russell; Danner, Raymond; Olsen, Brian; Luther, David (14 July 2011). "High summer temperature explains bill size variation in salt marsh sparrows". Ecography. online first (2): 146–152. doi:10.1111/j.1600-0587.2011.07002.x.
- Phillips, Polly K.; Sanborn, Allen F. (December 1994). "An infrared, thermographic study of surface temperature in three ratites: ostrich, emu and double-wattled cassowary". Journal of Thermal Biology. 19 (6): 423–430. doi:10.1016/0306-4565(94)90042-6.
- "Evolution of Bird Bills: Birds Reduce Their 'Heating Bills' in Cold Climates". Science Daily. 23 June 2010. Retrieved 12 March 2012.
- Bierma, Nathan (12 August 2004). "Add this to life list: 'Birding' has inspired flock of words". Chicago Tribune. Retrieved 6 June 2011.
- Terres, John K. (1980). The Audubon Society Encyclopedia of North American Birds. New York: Alfred A. Knopf. ISBN 978-0-394-46651-4.
- Schreiber, Elizabeth Anne; Burger, Joanna, eds. (2002). Biology of Marine Birds. Boca Raton, FL: CRC Press. p. 325. ISBN 978-0-8493-9882-7.
- Armstrong 1965, p. 7.
- Wilson, Edward O. (1980). Sociobiology. Boston, MA: Harvard University Press. p. 227. ISBN 978-0-674-81624-4.
- Amerson, A. Binion (May 1967). "Incidence and Transfer of Rhinonyssidae (Acarina: Mesostigmata) in Sooty Terns (Sterna fuscata)". Journal of Medical Entomology. 4 (2): 197–9. doi:10.1093/jmedent/4.2.197. PMID 6052126.
- Park, F. J. (March 2011). "Avian trichomoniasis: A study of lesions and relative prevalence in a variety of captive and free-living bird species as seen in an Australian avian practice". The Journal of the Australia Veterinary Association LTD. 89 (3): 82–88. doi:10.1111/j.1751-0813.2010.00681.x. PMID 21323655.
- Partridge, Eric (2001). Shakespeare's Bawdy (4 ed.). London: Routledge Classics 2001. p. 82. ISBN 978-0-415-25553-0.
- Burton, Maurice; Burton, Robert (1980). The International Wildlife Encyclopedia, volume 12. New York: Marshall Cavendish Corp. p. 1680.
- Grandin, Temple (2010). Improving Animal Welfare: A Practical Approach. Oxfordshire, UK: CABI. p. 110. ISBN 978-1-84593-541-2.
- "Bird Beaks: Anatomy, Care, and Diseases". Veterinary & Aquatic Services Department, Drs. Foster & Smith. Archived from the original on 4 June 2012. Retrieved 16 April 2012.
- Ash, Lydia. "Coping your Raptor". The Modern Apprentice. Retrieved 16 April 2012.
- Cunningham, Susan J.; Alley, M. R.; Castro, I.; Potter, M. A.; Cunningham, M.; Pyne, M. J. (2010). "Bill morphology or Ibises suggests a remote-tactile sensory system for prey detection". The Auk. 127 (2): 308–316. doi:10.1525/auk.2009.09117.
- Demery, Zoe P.; Chappell, J.; Martin, G. R. (2011). "Vision, touch and object manipulation in Senegal parrots Poicephalus senegalus". Proceedings of the Royal Society B. 278 (1725): 3687–3693. doi:10.1098/rspb.2011.0374. PMC 3203496. PMID 21525059.
Sources
| Wikimedia Commons has media related to Beaks. |
- Armstrong, Edward Allworthy (1965). Bird Display and Behaviour: An Introduction to the Study of Bird Psychology. New York, NY, US: Dover Publications. LCCN 64013457.
- Campbell, Bruce; Lack, Elizabeth, eds. (1985). A Dictionary of Birds. Carlton, England: T and A D Poyser. ISBN 978-0-85661-039-4.
- Coues, Elliott (1890). Handbook of Field and General Ornithology. London: Macmillan and Co. p. 1. OCLC 263166207.
- Gilbertson, Lance (1999). Zoology Lab Manual (4 ed.). New York: McGraw Hill Companies. ISBN 978-0-07-237716-3.
- Gill, Frank B. (1995). Ornithology (2 ed.). New York, NY: W. H. Freeman and Company. ISBN 978-0-7167-2415-5.
- Girling, Simon (2003). Veterinary Nursing of Exotic Pets. Oxford, UK: Blackwell Publishing. ISBN 978-1-4051-0747-1.
- Hill, Geoffrey E. (2010). National Geographic Bird Coloration. Washington, DC: National Geographic. ISBN 978-1-4262-0571-2.
- Howell, Steve N. G. (2007). Gulls of the Americas. New York: Houghton Mifflin Company. ISBN 978-0-618-72641-7.
- King, Anthony Stuart; McLelland, John, eds. (1985). Form and Function in Birds, volume 3. London, UK: Academic Press. ISBN 978-0-12-407503-0.
- Mullarney, Killian; Svensson, Lars; Zetterström, Dan; Grant, Peter J. (1999). Collins Bird Guide: The Most Complete Field Guide to the Birds of Britain and Europe. London: Harper Collins. ISBN 978-0-00-711332-3.
- Proctor, Noble S.; Lynch, Patrick J. (1998). Manual of Ornithology: Avian Structure and Function. New Haven, CT: Yale University Press. ISBN 978-0-300-07619-6.
- Rogers, Lesley J.; Kaplan, Gisela T. (2000). Songs, Roars and Rituals: Communication in Birds, Mammals and Other Animals. Boston, MA: Harvard University Press. ISBN 978-0-674-00827-4.
- Samour, Jaime, ed. (2000). Avian Medicine. London, UK: Mosby. ISBN 978-0-7234-2960-9.
