Feather
Feathers are epidermal growths that form distinctive outer covering, or plumage, on dinosaurs, both avian and some non-avian, and possibly other archosauromorphs. They are considered the most complex integumentary structures found in vertebrates[1][2] and a premier example of a complex evolutionary novelty.[3] They are among the characteristics that distinguish the extant birds from other living groups.[4]

Although feathers cover most of the bird's bodies, they arise only from certain well-defined tracts on the skin. They aid in flight, thermal insulation, and waterproofing. In addition, coloration helps in communication and protection.[5] Plumology (or plumage science) is the name for the science that is associated with the study of feathers.[6][7]
Structures and characteristics
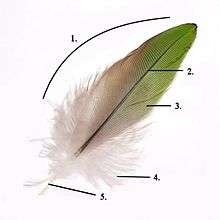
- Vane
- Rachis
- Barb
- Afterfeather
- Hollow shaft, calamus

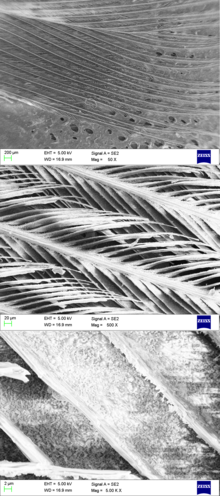
Feathers are among the most complex integumentary appendages found in vertebrates and are formed in tiny follicles in the epidermis, or outer skin layer, that produce keratin proteins. The β-keratins in feathers, beaks and claws — and the claws, scales and shells of reptiles — are composed of protein strands hydrogen-bonded into β-pleated sheets, which are then further twisted and crosslinked by disulfide bridges into structures even tougher than the α-keratins of mammalian hair, horns and hooves.[8][9] The exact signals that induce the growth of feathers on the skin are not known, but it has been found that the transcription factor cDermo-1 induces the growth of feathers on skin and scales on the leg.[10]
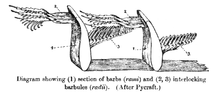
Classification
There are two basic types of feather: vaned feathers which cover the exterior of the body, and down feathers which are underneath the vaned feathers. The pennaceous feathers are vaned feathers. Also called contour feathers, pennaceous feathers arise from tracts and cover the entire body. A third rarer type of feather, the filoplume, is hairlike and (if present in a bird; they are entirely absent in ratites[11]) are closely associated with pennaceous feathers and are often entirely hidden by them, with one or two filoplumes attached and sprouting from near the same point of the skin as each pennaceous feather, at least on a bird's head, neck and trunk.[12][13] In some passerines, filoplumes arise exposed beyond the pennaceous feathers on the neck.[1] The remiges, or flight feathers of the wing, and rectrices, or flight feathers of the tail, are the most important feathers for flight. A typical vaned feather features a main shaft, called the rachis. Fused to the rachis are a series of branches, or barbs; the barbs themselves are also branched and form the barbules. These barbules have minute hooks called barbicels for cross-attachment. Down feathers are fluffy because they lack barbicels, so the barbules float free of each other, allowing the down to trap air and provide excellent thermal insulation. At the base of the feather, the rachis expands to form the hollow tubular calamus (or quill) which inserts into a follicle in the skin. The basal part of the calamus is without vanes. This part is embedded within the skin follicle and has an opening at the base (proximal umbilicus) and a small opening on the side (distal umbilicus).[14]
Hatchling birds of some species have a special kind of natal down feathers (neossoptiles) which are pushed out when the normal feathers (teleoptiles) emerge.[1]
Flight feathers are stiffened so as to work against the air in the downstroke but yield in other directions. It has been observed that the orientation pattern of β-keratin fibers in the feathers of flying birds differs from that in flightless birds: the fibers are better aligned along the shaft axis direction towards the tip,[15][16] and the lateral walls of rachis region show structure of crossed fibers.[17][18]
Functions
Feathers insulate birds from water and cold temperatures. They may also be plucked to line the nest and provide insulation to the eggs and young. The individual feathers in the wings and tail play important roles in controlling flight.[17] Some species have a crest of feathers on their heads. Although feathers are light, a bird's plumage weighs two or three times more than its skeleton, since many bones are hollow and contain air sacs. Color patterns serve as camouflage against predators for birds in their habitats, and serve as camouflage for predators looking for a meal. As with fish, the top and bottom colors may be different, in order to provide camouflage during flight. Striking differences in feather patterns and colors are part of the sexual dimorphism of many bird species and are particularly important in selection of mating pairs. In some cases there are differences in the UV reflectivity of feathers across sexes even though no differences in color are noted in the visible range.[19] The wing feathers of male club-winged manakins Machaeropterus deliciosus have special structures that are used to produce sounds by stridulation.[20]

Some birds have a supply of powder down feathers which grow continuously, with small particles regularly breaking off from the ends of the barbules. These particles produce a powder that sifts through the feathers on the bird's body and acts as a waterproofing agent and a feather conditioner. Powder down has evolved independently in several taxa and can be found in down as well as in pennaceous feathers. They may be scattered in plumage as in the pigeons and parrots or in localized patches on the breast, belly, or flanks, as in herons and frogmouths. Herons use their bill to break the powder down feathers and to spread them, while cockatoos may use their head as a powder puff to apply the powder.[21] Waterproofing can be lost by exposure to emulsifying agents due to human pollution. Feathers can then become waterlogged, causing the bird to sink. It is also very difficult to clean and rescue birds whose feathers have been fouled by oil spills. The feathers of cormorants soak up water and help to reduce buoyancy, thereby allowing the birds to swim submerged.[22]

Bristles are stiff, tapering feathers with a large rachis but few barbs. Rictal bristles are found around the eyes and bill. They may serve a similar purpose to eyelashes and vibrissae in mammals. Although there is as yet no clear evidence, it has been suggested that rictal bristles have sensory functions and may help insectivorous birds to capture prey.[23] In one study, willow flycatchers (Empidonax traillii) were found to catch insects equally well before and after removal of the rictal bristles.[24]
Grebes are peculiar in their habit of ingesting their own feathers and feeding them to their young. Observations on their diet of fish and the frequency of feather eating suggest that ingesting feathers, particularly down from their flanks, aids in forming easily ejectable pellets.[25]
Distribution
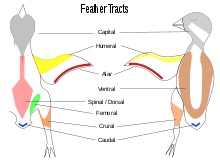
Contour feathers are not uniformly distributed on the skin of the bird except in some groups such as the penguins, ratites and screamers.[26] In most birds the feathers grow from specific tracts of skin called pterylae; between the pterylae there are regions which are free of feathers called apterylae (or apteria). Filoplumes and down may arise from the apterylae. The arrangement of these feather tracts, pterylosis or pterylography, varies across bird families and has been used in the past as a means for determining the evolutionary relationships of bird families.[27][28] Species that incubate their own eggs often lose their feathers on a region of their belly, forming a brooding patch.[29]
Coloration

Left: turacin (red) and turacoverdin (green, with some structural blue iridescence at lower end) on the wing of Tauraco bannermani
Right: carotenoids (red) and melanins (dark) on belly/wings of Ramphocelus bresilius
The colors of feathers are produced by pigments, by microscopic structures that can refract, reflect, or scatter selected wavelengths of light, or by a combination of both.
Most feather pigments are melanins (brown and beige pheomelanins, black and grey eumelanins) and carotenoids (red, yellow, orange); other pigments occur only in certain taxa – the yellow to red psittacofulvins[30] (found in some parrots) and the red turacin and green turacoverdin (porphyrin pigments found only in turacos).
Structural coloration[5][31][32] is involved in the production of blue colors, iridescence, most ultraviolet reflectance and in the enhancement of pigmentary colors. Structural iridescence has been reported[33] in fossil feathers dating back 40 million years. White feathers lack pigment and scatter light diffusely; albinism in birds is caused by defective pigment production, though structural coloration will not be affected (as can be seen, for example, in blue-and-white budgerigars).

The blues and bright greens of many parrots are produced by constructive interference of light reflecting from different layers of structures in feathers. In the case of green plumage, in addition to yellow, the specific feather structure involved is called by some the Dyck texture.[34][35] Melanin is often involved in the absorption of light; in combination with a yellow pigment, it produces a dull olive-green.
In some birds, feather colors may be created, or altered, by secretions from the uropygial gland, also called the preen gland. The yellow bill colors of many hornbills are produced by such secretions. It has been suggested that there are other color differences that may be visible only in the ultraviolet region,[21] but studies have failed to find evidence.[36] The oil secretion from the uropygial gland may also have an inhibitory effect on feather bacteria.[37]
The reds, orange and yellow colors of many feathers are caused by various carotenoids. Carotenoid-based pigments might be honest signals of fitness because they are derived from special diets and hence might be difficult to obtain,[38][39] and/or because carotenoids are required for immune function and hence sexual displays come at the expense of health.[40]
A bird's feathers undergo wear and tear and are replaced periodically during the bird's life through molting. New feathers, known when developing as blood, or pin feathers, depending on the stage of growth, are formed through the same follicles from which the old ones were fledged. The presence of melanin in feathers increases their resistance to abrasion.[41] One study notes that melanin based feathers were observed to degrade more quickly under bacterial action, even compared to unpigmented feathers from the same species, than those unpigmented or with carotenoid pigments.[42] However, another study the same year compared the action of bacteria on pigmentations of two song sparrow species and observed that the darker pigmented feathers were more resistant; the authors cited other research also published in 2004 that stated increased melanin provided greater resistance. They observed that the greater resistance of the darker birds confirmed Gloger's rule.[43]
Although sexual selection plays a major role in the development of feathers, in particular the color of the feathers it is not the only conclusion available. New studies are suggesting that the unique feathers of birds is also a large influence on many important aspects of avian behavior, such as the height at which a different species build their nests. Since females are the prime care givers, evolution has helped select females to display duller colored down so that they may blend into the nesting environment. The position of the nest and whether it has a greater chance of being under predation has exerted constraints on female birds' plumage.[44] A species of bird that nests on the ground, rather than the canopy of the trees, will need to have much duller colors in order not to attract attention to the nest. Since the female is the main care giver in some species of birds, evolution has helped select traits that make her feathers dull and often allow her to blend into the surroundings. The height study found that birds that nest in the canopies of trees often have many more predator attacks due to the brighter color of feathers that the female displays.[44] Another influence of evolution that could play a part in why feathers of birds are so colorful and display so many patterns could be due to that birds developed their bright colors from the vegetation and flowers that thrive around them. Birds develop their bright colors from living around certain colors. Most bird species often blend into their environment, due to some degree of camouflage, so if the species habitat is full of colors and patterns, the species would eventually evolve to blend in to avoid being eaten. Birds' feathers show a large range of colors, even exceeding the variety of many plants, leaf and flower colors.[45]
Parasites
The feather surface is the home for some ectoparasites, notably feather lice (Phthiraptera) and feather mites. Feather lice typically live on a single host and can move only from parents to chicks, between mating birds, and, occasionally, by phoresy. This life history has resulted in most of the parasite species being specific to the host and coevolving with the host, making them of interest in phylogenetic studies.[46]
Feather holes are chewing traces of lice (most probably Brueelia spp. lice) on the wing and tail feathers. They were described on barn swallows, and because of easy countability, many evolutionary, ecological, and behavioral publications use them to quantify the intensity of infestation.
Parasitic cuckoos which grow up in the nests of other species also have host-specific feather lice and these seem to be transmitted only after the young cuckoos leave the host nest.[47]
Birds maintain their feather condition by preening and bathing in water or dust. It has been suggested that a peculiar behavior of birds, anting, in which ants are introduced into the plumage, helps to reduce parasites, but no supporting evidence has been found.[48]
Human usage
Feathers have a number of utilitarian, cultural and religious uses.
Utilitarian functions


Feathers are both soft and excellent at trapping heat; thus, they are sometimes used in high-class bedding, especially pillows, blankets, and mattresses. They are also used as filling for winter clothing and outdoor bedding, such as quilted coats and sleeping bags. Goose and eider down have great loft, the ability to expand from a compressed, stored state to trap large amounts of compartmentalized, insulating air.[49]
Bird feathers have long been used for fletching arrows. Colorful feathers such as those belonging to pheasants have been used to decorate fishing lures.
Feathers of large birds (most often geese) have been and are used to make quill pens. The word pen itself is derived from the Latin penna, meaning feather.[50] The French word plume can mean either feather or pen.
Feathers are also valuable in aiding the identification of species in forensic studies, particularly in bird strikes to aircraft. The ratios of hydrogen isotopes in feathers help in determining the geographic origins of birds.[51] Feathers may also be useful in the non-destructive sampling of pollutants.[52]
The poultry industry produces a large amount of feathers as waste, which, like other forms of keratin, are slow to decompose. Feather waste has been used in a number of industrial applications as a medium for culturing microbes,[53] biodegradeable polymers,[54] and production of enzymes.[55] Feather proteins have been tried as an adhesive for wood board.[56]
Some groups of Native people in Alaska have used ptarmigan feathers as temper (non-plastic additives) in pottery manufacture since the first millennium BC in order to promote thermal shock resistance and strength.[57]
Historically, the hunting of birds for decorative and ornamental feathers (including in Victorian fashion) has endangered some species and helped to contribute to the extinction of others.[58] For instance, South American hummingbird feathers were used in the past to dress some of the miniature birds featured in singing bird boxes.
In religion and culture

Eagle feathers have great cultural and spiritual value to American Indians in the US and First Nations peoples in Canada as religious objects. In the United States the religious use of eagle and hawk feathers is governed by the eagle feather law, a federal law limiting the possession of eagle feathers to certified and enrolled members of federally recognized Native American tribes.
In South America, brews made from the feathers of condors are used in traditional medications.[59] In India, feathers of the Indian peacock have been used in traditional medicine for snakebite, infertility, and coughs.[60][61]
During the 18th, 19th, and early 20th centuries, there was a booming international trade in plumes for extravagant women's hats and other headgear. Frank Chapman noted in 1886 that feathers of as many as 40 species of birds were used in about three-fourths of the 700 ladies' hats that he observed in New York City.[62] This trade caused severe losses to bird populations (for example, egrets and whooping cranes). Conservationists led a major campaign against the use of feathers in hats. This contributed to passage of the Lacey Act in 1900, and to changes in fashion. The ornamental feather market then largely collapsed.[63][64]
More recently, rooster plumage has become a popular trend as a hairstyle accessory, with feathers formerly used as fishing lures now being used to provide color and style to hair.[65] Today, feathers used in fashion and in military headdresses and clothes are obtained as a waste product of poultry farming, including chickens, geese, turkeys, pheasants, and ostriches. These feathers are dyed and manipulated to enhance their appearance, as poultry feathers are naturally often dull in appearance compared to the feathers of wild birds.
Feather products manufacturing in Europe has declined in the last 60 years, mainly due to competition from Asia. Feathers have adorned hats at many prestigious events such as weddings and Ladies Day at racecourses (Royal Ascot).
Evolution
.jpg)
The functional view on the evolution of feathers has traditionally focused on insulation, flight and display. Discoveries of non-flying Late Cretaceous feathered dinosaurs in China,[66] however, suggest that flight could not have been the original primary function as the feathers simply would not have been capable of providing any form of lift.[67][68] There have been suggestions that feathers may have had their original function in thermoregulation, waterproofing, or even as sinks for metabolic wastes such as sulphur.[69] Recent discoveries are argued to support a thermoregulatory function, at least in smaller dinosaurs.[70][71] Some researchers even argue that thermoregulation arose from bristles on the face that were used as tactile sensors.[72] While feathers have been suggested as having evolved from reptilian scales, there are numerous objections to that idea, and more recent explanations have arisen from the paradigm of evolutionary developmental biology.[2] Theories of the scale-based origins of feathers suggest that the planar scale structure was modified for development into feathers by splitting to form the webbing; however, that developmental process involves a tubular structure arising from a follicle and the tube splitting longitudinally to form the webbing.[1][2] The number of feathers per unit area of skin is higher in smaller birds than in larger birds, and this trend points to their important role in thermal insulation, since smaller birds lose more heat due to the relatively larger surface area in proportion to their body weight.[5] The miniaturization of birds also played a role in the evolution of powered flight.[73] The coloration of feathers is believed to have evolved primarily in response to sexual selection. In one fossil specimen of the paravian Anchiornis huxleyi, the features are so well preserved that the melanosome (pigment cells) structure can be observed. By comparing the shape of the fossil melanosomes to melanosomes from extant birds, the color and pattern of the feathers on Anchiornis could be determined.[74] Anchiornis was found to have black-and-white-patterned feathers on the forelimbs and hindlimbs, with a reddish-brown crest. This pattern is similar to the coloration of many extant bird species, which use plumage coloration for display and communication, including sexual selection and camouflage. It is likely that non-avian dinosaur species utilized plumage patterns for similar functions as modern birds before the origin of flight. In many cases, the physiological condition of the birds (especially males) is indicated by the quality of their feathers, and this is used (by the females) in mate choice.[75][76] Additionally, when comparing different Ornithomimus edmontonicus specimens, older individuals were found to have a pennibrachium (a wing-like structure consisting of elongate feathers), while younger ones did not. This suggests that the pennibrachium was a secondary sex characteristic and likely had a sexual function.[77]
Feathers and scales are made up of two distinct forms of keratin, and it was long thought that each type of keratin was exclusive to each skin structure (feathers and scales). However, a study published in 2006 confirmed the presence of feather keratin in the early stages of development of American alligator scales. This type of keratin, previously thought to be specific to feathers, is suppressed during embryological development of the alligator and so is not present in the scales of mature alligators. The presence of this homologous keratin in both birds and crocodilians indicates that it was inherited from a common ancestor. This may suggest that crocodilian scales, bird and dinosaur feathers, and pterosaur pycnofibres are all developmental expressions of the same primitive archosaur skin structures; suggesting that feathers and pycnofibers could be homologous.[78]
Feathered dinosaurs
.jpg)
Several non-avian dinosaurs had feathers on their limbs that would not have functioned for flight.[66][2] One theory suggests that feathers originally evolved on dinosaurs due to their insulation properties; then, small dinosaur species which grew longer feathers may have found them helpful in gliding, leading to the evolution of proto-birds like Archaeopteryx and Microraptor zhaoianus. Another theory posits that the original adaptive advantage of early feathers was their pigmentation or iridescence, contributing to sexual preference in mate selection.[79] Dinosaurs that had feathers or protofeathers include Pedopenna daohugouensis[80] and Dilong paradoxus, a tyrannosauroid which is 60 to 70 million years older than Tyrannosaurus rex.[81]
The majority of dinosaurs known to have had feathers or protofeathers are theropods, however featherlike "filamentous integumentary structures" are also known from the ornithischian dinosaurs Tianyulong and Psittacosaurus.[82] The exact nature of these structures is still under study. However, it is believed that the stage-1 feathers (see Evolutionary stages section below) such as those seen in these two ornithischians likely functioned in display.[83] In 2014, the ornithischian Kulindadromeus was reported as having structures resembling stage-3 feathers.[84]
Since the 1990s, dozens of feathered dinosaurs have been discovered in the clade Maniraptora, which includes the clade Avialae and the recent common ancestors of birds, Oviraptorosauria and Deinonychosauria. In 1998, the discovery of a feathered oviraptorosaurian, Caudipteryx zoui, challenged the notion of feathers as a structure exclusive to Avialae.[85] Buried in the Yixian Formation in Liaoning, China, C. zoui lived during the Early Cretaceous Period. Present on the forelimbs and tails, their integumentary structure has been accepted as pennaceous vaned feathers based on the rachis and herringbone pattern of the barbs. In the clade Deinonychosauria, the continued divergence of feathers is also apparent in the families Troodontidae and Dromaeosauridae. Branched feathers with rachis, barbs, and barbules were discovered in many members including Sinornithosaurus millenii, a dromaeosaurid found in the Yixian formation (124.6 MYA).[86]
Previously, a temporal paradox existed in the evolution of feathers—theropods with highly derived bird-like characteristics occurred at a later time than Archaeopteryx—suggesting that the descendants of birds arose before the ancestor. However, the discovery of Anchiornis huxleyi in the Late Jurassic Tiaojishan Formation (160 MYA) in western Liaoning in 2009[87][88] resolved this paradox. By predating Archaeopteryx, Anchiornis proves the existence of a modernly feathered theropod ancestor, providing insight into the dinosaur-bird transition. The specimen shows distribution of large pennaceous feathers on the forelimbs and tail, implying that pennaceous feathers spread to the rest of the body at an earlier stage in theropod evolution.[89] The development of pennaceous feathers did not replace earlier filamentous feathers. Filamentous feathers are preserved alongside modern-looking flight feathers — including some with modifications found in the feathers of extant diving birds — in 80 million year old amber from Alberta.[90]
Two small wings trapped in amber dating to 100 mya show plumage existed in some bird predecessors. The wings most probably belonged to enantiornithes, a diverse group of avian dinosaurs.[91][92]
A large phylogenetic analysis of early dinosaurs by Matthew Baron, David B. Norman and Paul Barrett (2017) found that Theropoda is actually more closely related to Ornithischia, to which it formed the sister group within the clade Ornithoscelida. The study also suggested that if the feather-like structures of theropods and ornithischians are of common evolutionary origin then it would be possible that feathers were restricted to Ornithoscelida. If so, then the origin of feathers would have likely occurred as early as the Middle Triassic.[93]
Evolutionary stages
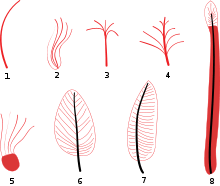
Several studies of feather development in the embryos of modern birds, coupled with the distribution of feather types among various prehistoric bird precursors, have allowed scientists to attempt a reconstruction of the sequence in which feathers first evolved and developed into the types found on modern birds.
Feather evolution was broken down into the following stages by Xu and Guo in 2009:[83]
- Single filament
- Multiple filaments joined at their base
- Multiple filaments joined at their base to a central filament
- Multiple filaments along the length of a central filament
- Multiple filaments arising from the edge of a membranous structure
- Pennaceous feather with vane of barbs and barbules and central rachis
- Pennaceous feather with an asymmetrical rachis
- Undifferentiated vane with central rachis
However, Foth (2011) showed that some of these purported stages (stages 2 and 5 in particular) are likely simply artifacts of preservation caused by the way fossil feathers are crushed and the feather remains or imprints are preserved. Foth re-interpreted stage 2 feathers as crushed or misidentified feathers of at least stage 3, and stage 5 feathers as crushed stage 6 feathers.[94]
The following simplified diagram of dinosaur relationships follows these results, and shows the likely distribution of plumaceous (downy) and pennaceous (vaned) feathers among dinosaurs and prehistoric birds. The diagram follows one presented by Xu and Guo (2009)[83] modified with the findings of Foth (2011).[94] The numbers accompanying each name refer to the presence of specific feather stages. Note that 's' indicates the known presence of scales on the body.
See also
- Feather development
- Delayed feathering in chickens
- Hen feathering in cocks
- List of poultry feathers
- Pinioning
- Plumage
- White feather
References
- Prum, Richard O. & AH Brush (2002). "The evolutionary origin and diversification of feathers" (PDF). The Quarterly Review of Biology. 77 (3): 261–295. doi:10.1086/341993. PMID 12365352. Archived (PDF) from the original on 29 June 2011. Retrieved 7 July 2010.CS1 maint: uses authors parameter (link)
- Prum, R.O. & Brush, A.H (March 2003). "Which Came First, the Feather or the Bird?" (PDF). Scientific American. 288 (3): 84–93. Bibcode:2003SciAm.288c..84P. doi:10.1038/scientificamerican0303-84. PMID 12616863. Archived (PDF) from the original on 29 June 2011. Retrieved 7 July 2010.
- Prum, Richard O (1999). "Development and Evolutionary Origin of Feathers" (PDF). Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 285 (4): 291–306. doi:10.1002/(SICI)1097-010X(19991215)285:4<291::AID-JEZ1>3.0.CO;2-9. PMID 10578107. Archived from the original (PDF) on 9 April 2011. Retrieved 7 July 2010.
- Li, Quanguo (9 March 2012). "Reconstruction of Microraptor and the Evolution of Iridescent Plumage". Science. 335 (6073): 1215–1219. Bibcode:2012Sci...335.1215L. doi:10.1126/science.1213780. PMID 22403389. S2CID 206537426.
- Pettingill, OS Jr. (1970). Ornithology in Laboratory and Field. Fourth edition. Burgess Publishing Company. pp. 29–58. ISBN 0808716093.
- "Galapagos plumology" (PDF). darwinfoundation.org. Charles Darwin Collections Database by the Charles Darwin Foundation. Archived from the original on 17 March 2016. Retrieved 24 April 2015.
- Eichhorn, hrsg. von Manfred (2005). Langenscheidt Fachwörterbuch Biologie Englisch : englisch - deutsch, deutsch - englisch (1. Aufl. ed.). Berlin [u.a.]: Langenscheidt. p. 537. ISBN 3861172283. Archived from the original on 17 December 2017. Retrieved 24 April 2015.
- Schor, R.; Krimm, S. (1961). "Studies on the Structure of Feather Keratin: II. A β-Helix Model for the Structure of Feather Keratin". Biophys. J. 1 (6): 489–515. Bibcode:1961BpJ.....1..489S. doi:10.1016/S0006-3495(61)86904-X. PMC 1366335. PMID 19431311.
- Pauling, Linus; Corey, Robert B. (1951). "The Structure of Feather Rachis Keratin". Proceedings of the National Academy of Sciences of the United States of America. 37 (5): 256–261. Bibcode:1951PNAS...37..256P. doi:10.1073/pnas.37.5.256. PMC 1063351. PMID 14834148.
- Hornik, C.; Krishan, K.; Yusuf, F.; Scaal, M.; Brand-Saberi, B. (2005). "cDermo-1 misexpression induces dense dermis, feathers, and scales". Developmental Biology. 277 (1): 42–50. doi:10.1016/j.ydbio.2004.08.050. PMID 15572138.
- Chandler, A. C. (1916). A study of the structure of feathers, with reference to their taxonomic significance. Berkeley: University of California. p. 285.
- Nitzsch, Christian Ludwig (1867). Nitzsch's Pterylography. Ray Society. p. 14.
- Chandler 1916, p. 261
- McLelland, J. (1991). A color atlas of avian anatomy. W.B. Saunders Co. ISBN 0-7216-3536-9.
- Cameron, G.; Wess, T.; Bonser, R. (2003). "Young's modulus varies with differential orientation of keratin in feathers". Journal of Structural Biology. 143 (2): 118–23. doi:10.1016/S1047-8477(03)00142-4. PMID 12972348.
- Bonser, R.; Saker, L.; Jeronimidis, G. (2004). "Toughness anisotropy in feather keratin". Journal of Materials Science. 39 (8): 2895–2896. Bibcode:2004JMatS..39.2895B. doi:10.1023/B:JMSC.0000021474.75864.ff.
- Wang, Bin (2016). "Light like a feather: A fibrous natural composite with a shape changing from round to square". Advanced Science. 4 (3): 1600360. doi:10.1002/advs.201600360. PMC 5357985. PMID 28331789.
- Lingham-Soliar, Theagarten (2013). "A new helical crossed-fibre structure of b-keratin in flight feathers and its biomechanical implications". PLOS ONE. 8 (6): e65849. Bibcode:2013PLoSO...865849L. doi:10.1371/journal.pone.0065849. PMC 3677936. PMID 23762440.
- Eaton, Muir D.; Lanyon, Scott M. (2003). "The ubiquity of avian ultraviolet plumage reflectance". Proceedings: Biological Sciences. 270 (1525): 1721–1726. doi:10.1098/rspb.2003.2431. PMC 1691429. PMID 12965000.
- Bostwick, Kimberly S.; Richard O., Prum (2005). "Courting Bird Sings with Stridulating Wing Feathers" (PDF). Science. 309 (5735): 736. doi:10.1126/science.1111701. PMID 16051789. Archived (PDF) from the original on 7 July 2010. Retrieved 19 July 2010.
- Delhey, K; Peters, A.; Kempenaers, B. (2007). "Cosmetic coloration in birds: occurrence, function and evolution" (PDF). Am. Nat. 169: S145–158. doi:10.1086/510095. PMID 19426089. Archived from the original (PDF) on 3 December 2007.
- Ribak, G.; Weihs, D.; Arad, Z. (2005). "Water retention in the plumage of diving great cormorants Phalacrocorax carbo sinensis". J. Avian Biol. 36 (2): 89–95. doi:10.1111/j.0908-8857.2005.03499.x.
- Lederer, Roger J. (1972). "The role of avian rictal bristles" (PDF). The Wilson Bulletin. 84: 193–97. Archived (PDF) from the original on 4 February 2014.
- Conover, M. R.; Miller, D. E. (1980). "Rictal bristle function in willow flycatcher". Condor. 82 (4): 469–471. doi:10.2307/1367580. JSTOR 1367580. Archived from the original on 22 February 2014.
- Piersma, T; van Eerden, M. R. (1989). "Feather eating in Great Crested Grebes Podiceps cristatus: a unique solution to the problems of debris and gastric parasites in fish-eating birds". Ibis. 131 (4): 477–486. doi:10.1111/j.1474-919X.1989.tb04784.x.
- Demay, Ida S. (1940). "A Study of the Pterylosis and Pneumaticity of the Screamer". The Condor. 42 (2): 112–118. doi:10.2307/1364475. JSTOR 1364475. Archived from the original on 21 February 2014.
- Hall, K.; Susanna S. (2005). "Do nine-primaried passerines have nine or ten primary feathers? The evolution of a concept". Journal of Ornithology. 146 (2): 121–126. doi:10.1007/s10336-004-0070-5.
- Pycraft, W. P. (1895). "On the pterylography of the hoatzin (Opisthocomus cristatus)". Ibis. 37 (3): 345–373. doi:10.1111/j.1474-919X.1895.tb06744.x.
- Turner, J. Scott (1997). "On the Thermal Capacity of a Bird's Egg Warmed by a Brood Patch" (PDF). Physiological Zoology. 70: 470–80 – via EBSCO.
- McGraw, KH; Nogare, MC (2005). "Distribution of unique red feather pigments in parrots". Biology Letters. 1 (1): 38–43. doi:10.1098/rsbl.2004.0269. PMC 1629064. PMID 17148123.
- Hausmann, F.; Arnold, K.E.; Marshall, N.J.; Owens, I.P.F. (2003). "Ultraviolet signals in birds are special". Proceedings of the Royal Society B. 270 (1510): 61–67. doi:10.1098/rspb.2002.2200. PMC 1691211. PMID 12590772.
- Shawkey, Matthew D; Hill, Geoffrey E (2005). "Carotenoids need structural colours to shine" (PDF). Biol. Lett. 1 (2): 121–124. doi:10.1098/rsbl.2004.0289. PMC 1626226. PMID 17148144. Archived from the original (PDF) on 26 March 2009.
- Vinther, Jakob; Briggs, Derek E. G.; Clarke, Julia; Mayr, Gerald; Prum, Richard O. (2009). "Structural coloration in a fossil feather" (PDF). Biology Letters. 6 (1): 128–31. doi:10.1098/rsbl.2009.0524. PMC 2817243. PMID 19710052. Archived from the original (PDF) on 21 June 2010. Retrieved 19 July 2010.
- Dyck, J. (1971). "Structure and spectral reflectance of green and blue feathers of the Lovebird (Agapornis roseicollis)". Biol. SKR. 18: 1–67.
- Shawkey, M. D.; Hill, G. E. (2005). "Feathers at a fine scale" (PDF). The Auk. 121 (3): 652–655. doi:10.1642/0004-8038(2004)121[0652:FAAFS]2.0.CO;2. Archived from the original (PDF) on 26 March 2009.
- Delhey, K.; Peters, A.; Biedermann, P. H. W.; Kempenaers, B. (2008). "Optical properties of the uropygial gland secretion: no evidence for UV cosmetics in birds". Naturwissenschaften. 95 (10): 939–46. Bibcode:2008NW.....95..939D. doi:10.1007/s00114-008-0406-8. PMID 18560743.
- Shawkey, M. D.; Pillai, S. R.; Hill, G. E. (2003). "Chemical warfare? Effects of uropygial oil on feather-degrading bacteria" (PDF). Journal of Avian Biology. 34 (4): 345–349. doi:10.1111/j.0908-8857.2003.03193.x. Archived from the original (PDF) on 10 September 2008.
- Endler, J. A. (1980). "Natural selection on color patterns in Poeci-lia reticulata". Evolution. 34 (1): 76–91. doi:10.2307/2408316. JSTOR 2408316. PMID 28563214.
- Badyaev, A. V.; Hill, G. E. (2000). "Evolution of sexual dichromatism: contribution of carotenoid versus melanin-based colouration". Biological Journal of the Linnean Society. 69 (2): 153–172. doi:10.1111/j.1095-8312.2000.tb01196.x.
- Lozano, G. A. (1994). "Carotenoids, parasites, and sexual selection". Oikos. 70 (2): 309–311. doi:10.2307/3545643. JSTOR 3545643. S2CID 86971117.
- Bonser, R. H. C. (1995). "Melanin and the abrasion resistance of feathers". Condor. 97 (2): 590–591. doi:10.2307/1369048. JSTOR 1369048. Archived from the original on 23 February 2014.
- Grande, J. M.; Negro, J. J.; Torres, M. J. (2004). "The evolution of bird plumage colouration: A role for feather-degrading bacteria?" (PDF). Ardeola. 51 (2): 375–383. Archived (PDF) from the original on 10 September 2008.
- Burtt, Edward H. Jr.; Ichida, Jann M. (2004). "Gloger's Rule, feather-degrading bacteria, and color variation among Song Sparrows" (PDF). Condor. 106 (3): 681–686. doi:10.1650/7383. Archived (PDF) from the original on 20 November 2012.
- Martin, T. E.; Badyaev, A. V. (1996). "Sexual dichromatic in birds; importance of nest predation and nest location for females versus males". Evolution. 50 (6): 2454–2460. doi:10.2307/2410712. JSTOR 2410712.
- Caswell Stoddard, Mary; Prum, Richard O. (2011). "How colorful are birds? Evolution of the avian plumage color gamut". Behavioral Ecology. 22 (5): 1042–1052. doi:10.1093/beheco/arr088.
- Toon, A. & Hughes, J. (2008). "Are lice good proxies for host history? A comparative analysis of the Australian magpie, Gymnorhina tibicen, and two species of feather louse". Heredity. 101 (2): 127–135. doi:10.1038/hdy.2008.37. PMID 18461081.
- Brooke, M. de L.; Hiroshi Nakamura (1998). "The acquisition of host-specific feather lice by common cuckoos (Cuculus canorus)". Journal of Zoology. 244 (2): 167–173. doi:10.1017/S0952836998002027.
- Revis, Hannah C.; Deborah A. Waller (2004). "Bactericidal and fungicidal activity of ant chemicals on feather parasites: an evaluation of anting behavior as a method of self-medication in songbirds". Auk. 121 (4): 1262–1268. doi:10.1642/0004-8038(2004)121[1262:BAFAOA]2.0.CO;2.
- Bonser, R.H.C.; Dawson, C. (1999). "The structural mechanical properties of down feathers and biomimicking natural insulation materials". Journal of Materials Science Letters. 18 (21): 1769–1770. doi:10.1023/A:1006631328233.
- "pen(3)". The Merriam-Webster Online Dictionary. Merriam-Webster, Inc. Archived from the original on 19 September 2011. Retrieved 16 October 2010.
- Bowen, Gabriel J; Wassenaar, Leonard I; Hobson, Keith A (2005). "Global application of stable hydrogen and oxygen isotopes to wildlife forensics". Oecologia. 143 (3): 337–348. Bibcode:2005Oecol.143..337B. doi:10.1007/s00442-004-1813-y. PMID 15726429.
- Jaspers, V.; Voorspoels, S.; Covaci, A.; Lepoint, G. & Eens, M. (2007). "Evaluation of the usefulness of bird feathers as a non-destructive biomonitoring tool for organic pollutants: A comparative and meta-analytical approach". Environment International. 33 (3): 328–337. doi:10.1016/j.envint.2006.11.011. PMID 17198730.
- Poopathi, S.; Abidha, S. (2007). "Use of feather-based culture media for the production of mosquitocidal bacteria". Biological Control. 43 (1): 49–55. doi:10.1016/j.biocontrol.2007.04.019.
- Schmidt, W.F.; Barone, J.R. (2004). "New uses for chicken feathers keratin fiber. Poultry Waste Management Symposium Proceedings": 99–101. Cite journal requires
|journal=(help) - Casarin, Franciani; Brandelli, Florencia Cladera-Olivera Adriano; Brandelli, Adriano (2008). "Use of Poultry Byproduct for Production of Keratinolytic Enzymes". Food and Bioprocess Technology. 1 (3): 301–305. doi:10.1007/s11947-008-0091-9.
- Jiang, Z.; Qin, D.; Hse, C.; Kuo, M.; Luo, Z.; Wang, G.; et al. (2008). "Preliminary Study on Chicken Feather Protein-Based Wood Adhesives". Journal of Wood Chemistry & Technology. 28 (3): 240–246. doi:10.1080/02773810802347073. Archived from the original on 19 February 2014.
- Neusius, Sarah W. and G. Timothy Gross 2007 Seeking Our Past: An Introduction to North American Archaeology. Oxford University Press, NY.
- Johnston, Nicole & Parsons, Jean (20 September 2018). "Feathers: Endangered – Fauna and Fashion". University of Missouri's Historic Costume and Textiles Collection.
- Froemming, Steve (2006). "Traditional use of the Andean flicker (Colaptes rupicola) as a galactagogue in the Peruvian Andes". Journal of Ethnobiology and Ethnomedicine. 2: 23. doi:10.1186/1746-4269-2-23. PMC 1484469. PMID 16677398.
- Murari, S.K.; Frey, F.J.; Frey, B.M.; Gowda, T.V.; Vishwanath, B.S. (2005). "Use of Pavo cristatus feather extract for the better management of snakebites: Neutralization of inflammatory reactions". Journal of Ethnopharmacology. 99 (2): 229–237. doi:10.1016/j.jep.2005.02.027. PMID 15894132.
- Mahawar, M. M.; Jaroli, D. P. (2007). "Traditional knowledge on zootherapeutic uses by the Saharia tribe of Rajasthan, India". Journal of Ethnobiology and Ethnomedicine. 3: 25. doi:10.1186/1746-4269-3-25. PMC 1892771. PMID 17547781.
- Doughty, Robin W. Feather Fashions and Bird Preservation, A Study in Nature Protection. University of California Press. Page 197.
- Ehrlich, Paul R.; Dobkin, David S.; Wheye, Darryl (1988). "Plume Trade". Stanford University. Archived from the original on 30 September 2008.
- Feather trade Archived 23 June 2008 at the Wayback Machine, Smithsonian Institution
- Bonner, Jessie L. (6 June 2011). "High fashion or bait? Fly ties now hair extensions". The Seattle Times. Archived from the original on 10 June 2011.
- St. Fleur, Nicholas (8 December 2016). "That Thing With Feathers Trapped in Amber? It Was a Dinosaur Tail". The New York Times. Archived from the original on 8 December 2016. Retrieved 8 December 2016.
- Sumida, SS; CA Brochu (2000). "Phylogenetic context for the origin of feathers". American Zoologist. 40 (4): 486–503. doi:10.1093/icb/40.4.486. Archived from the original on 29 August 2008.
- Dimond, C. C., R. J. Cabin and J. S. Brooks (2011). "Feathers, Dinosaurs, and Behavioral Cues: Defining the Visual Display Hypothesis for the Adaptive Function of Feathers in Non-Avian Theropods". BIOS. 82 (3): 58–63. doi:10.1893/011.082.0302.CS1 maint: uses authors parameter (link)
- Bock, WJ (2000). "Explanatory History of the Origin of Feathers". Am. Zool. 40 (4): 478–485. doi:10.1093/icb/40.4.478.
- Whitfield, John (4 April 2012). "Largest feathered dinosaur yet discovered in China". Nature News Blog. Archived from the original on 6 April 2012. Retrieved 4 April 2012.
- Xu X.; Wang K.; Zhang K.; Ma Q.; Xing L.; Sullivan C.; Hu D.; Cheng S.; Wang S.; et al. (2012). "A gigantic feathered dinosaur from the Lower Cretaceous of China" (PDF). Nature. 484 (7392): 92–95. Bibcode:2012Natur.484...92X. doi:10.1038/nature10906. PMID 22481363. Archived from the original (PDF) on 17 April 2012.
- Persons, Walter S.; Currie, Philip J. (2015). "Bristles before down: A new perspective on the functional origin of feathers". Evolution. 69 (4): 857–862. doi:10.1111/evo.12634. ISSN 1558-5646. PMID 25756292.
- De Ricqles; A. J.; K. Padian; J. R. Horner; E. T. Lamm; N. Myhrvold (2003). "Osteohistology of confuciusornis sanctus (theropoda: Aves)". Journal of Vertebrate Paleontology. 23 (2): 373–386. doi:10.1671/0272-4634(2003)023[0373:oocsta]2.0.co;2.
- Li, Quanguo; Gao, Ke-Qin; Vinther, Jakob; Shawkey, Matthew; Clarke, Julia; D'Alba, Liliana; Meng, Qingjin; Briggs, Derek; Prum, Richard (12 March 2010). "Plumage Color Patterns of an Extinct Dinosaur" (PDF). Science. 327 (5971): 1369–1372. Bibcode:2010Sci...327.1369L. doi:10.1126/science.1186290. PMID 20133521.
- Saino, Nicola; Riccardo Stradi (1999). "Carotenoid Plasma Concentration, Immune Profile, and Plumage Ornamentation of Male Barn Swallows". American Naturalist. 154 (4): 441–448. doi:10.1086/303246. PMID 10523490. S2CID 4400888.
- Endler, John A.; David A. Westcott; Joah R. Madden; Tim Robson & Patrick Phillips (2005). "Animal visual systems and the evolution of color patterns: Sensory processing illumiates signal evolution". Evolution. 59 (8): 1795–1818. doi:10.1111/j.0014-3820.2005.tb01827.x. PMID 16329248.
- Zelenitsky, D. K.; Therrien, F.; Erickson, G. M.; DeBuhr, C. L.; Kobayashi, Y.; Eberth, D. A.; Hadfield, F. (26 October 2012). "Feathered Non-Avian Dinosaurs from North America Provide Insight into Wing Origins". Science. 338 (6106): 510–514. Bibcode:2012Sci...338..510Z. doi:10.1126/science.1225376. ISSN 0036-8075. PMID 23112330.
- Alibardi, L; Knapp, LW; Sawyer, RH (2006). "Beta-keratin localization in developing alligator scales and feathers in relation to the development and evolution of feathers". Journal of Submicroscopic Cytology and Pathology. 38 (2–3): 175–92. PMID 17784647.
- Dimond, C. C., R. J. Cabin and J. S. Brooks (2011). "Feathers, Dinosaurs, and Behavioral Cues: Defining the Visual Display Hypothesis for the Adaptive Function of Feathers in Non-Avian Theropods". BIOS. 82 (3): 58–63. doi:10.1893/011.082.0302.CS1 maint: uses authors parameter (link)
- Xu, Xing; Fucheng Zhang (2005). "A new maniraptoran dinosaur from China with long feathers on the metatarsus". Naturwissenschaften. 92 (4): 173–177. Bibcode:2005NW.....92..173X. doi:10.1007/s00114-004-0604-y. PMID 15685441.
- Xu, Xing (2006). "Feathered dinosaurs from China and the evolution of major avian characters". Integrative Zoology. 1 (1): 4–11. doi:10.1111/j.1749-4877.2006.00004.x. PMID 21395983. S2CID 1516713.
- Zheng, X. T.; H. L. You; X. Xu & Z. M. Dong (2009). "An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures". Nature. 458 (7236): 333–336. Bibcode:2009Natur.458..333Z. doi:10.1038/nature07856. PMID 19295609.
- Xu, X.; Guo, Y. (2009). "The origin and early evolution of feathers: insights from recent paleontological and neontological data". Vertebrata PalAsiatica. 47 (4): 311–329.
- Godefroit, Pascal; Sinitsa, Sofia M.; Dhouailly, Danielle; Bolotsky, Yuri L.; Sizov, Alexander V.; McNamara, Maria E.; Benton, Michael J.; Spagna, Paul (2014). "A Jurassic ornithischian dinosaur from Siberia with both feathers and scales". Science. 345 (6195): 451–455. Bibcode:2014Sci...345..451G. doi:10.1126/science.1253351. PMID 25061209.
- Ji, Q., P. J. Currie, M. A. Norell, and S. A. Ji (1998). "Two feathered dinosaurs from northeastern China". Nature. 393 (6687): 753–761. Bibcode:1998Natur.393..753Q. doi:10.1038/31635.CS1 maint: uses authors parameter (link)
- Xu, X.; H. H. Zhou & R. O. Prum (2001). "Branched integumental structures in Sinornithosaurus and the origin of feathers". Nature. 410 (6825): 200–204. Bibcode:2001Natur.410..200X. doi:10.1038/35065589. PMID 11242078.
- Hu, D. Y., L. H. Hou, L. J. Zhang, and X. Xu (2009). "A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus". Nature. 461 (7264): 640–643. Bibcode:2009Natur.461..640H. doi:10.1038/nature08322. PMID 19794491.CS1 maint: uses authors parameter (link)
- Xu, X.; Q. Zhao; M. Norell; C. Sullivan; D. Hone; G. Erickson; X. L. Wang; et al. (2009). "A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin". Chinese Science Bulletin. 54 (3): 430–435. doi:10.1007/s11434-009-0009-6.
- Witmer, L. M. (2009). "Feathered dinosaurs in a tangle". Nature. 461 (7264): 601–602. Bibcode:2009Natur.461..601W. doi:10.1038/461601a. PMID 19794481.
- "Dinosaur feathers found in Alberta amber". CBC News. 15 September 2011. Archived from the original on 15 September 2011.
- "Rare Dinosaur-Era Bird Wings Found Trapped in Amber". 28 June 2016. Archived from the original on 28 June 2016. Retrieved 28 June 2016.
- Xing, Lida; McKellar, Ryan C.; Wang, Min; Bai, Ming; O’Connor, Jingmai K.; Benton, Michael J.; Zhang, Jianping; Wang, Yan; Tseng, Kuowei (28 June 2016). "Mummified precocial bird wings in mid-Cretaceous Burmese amber". Nature Communications. 7: 12089. Bibcode:2016NatCo...712089X. doi:10.1038/ncomms12089. PMC 4931330. PMID 27352215.
- Baron, M.G.; Norman, D.B.; Barrett, P.M. (2017). "A new hypothesis of dinosaur relationships and early dinosaur evolution". Nature. 543 (7646): 501–506. Bibcode:2017Natur.543..501B. doi:10.1038/nature21700. PMID 28332513.
- Foth, C (2011). "On the identification of feather structures in stem-line representatives of birds: evidence from fossils and actuopalaeontology". Paläontologische Zeitschrift. 86: 91–102. doi:10.1007/s12542-011-0111-3.
- Benton, Michael J.; Xu, Xing; Orr, Patrick J.; Kaye, Thomas G.; Pittman, Michael; Kearns, Stuart L.; McNamara, Maria E.; Jiang, Baoyu; Yang, Zixiao (January 2019). "Pterosaur integumentary structures with complex feather-like branching" (PDF). Nature Ecology & Evolution. 3 (1): 24–30. doi:10.1038/s41559-018-0728-7. hdl:1983/1f7893a1-924d-4cb3-a4bf-c4b1592356e9. ISSN 2397-334X. PMID 30568282.
- "Pterosaurs Had Four Types of Feathers, New Study Shows | Paleontology | Sci-News.com". Breaking Science News | Sci-News.com. Retrieved 19 December 2018.
- St. Fleur, Nicholas (17 December 2018). "Feathers and Fur Fly Over Pterosaur Fossil Finding - An analysis of two fossils would push back the origins of feathers by about 70 million years, but more specimens may be needed for confirmation". The New York Times. Retrieved 19 December 2018.
- Briggs, Helen (17 December 2018). "Fur flies over new pterosaur fossils". BBC News. Retrieved 19 December 2018.
Further reading
- Hanson, Thor (2011). Feathers: The Evolution of a Natural Miracle. New York: Basic Books. ISBN 9780465020133. OCLC 727106416.
External links
| Wikimedia Commons has media related to Feather. |
| Look up pluma or calamus in Wiktionary, the free dictionary. |
- Chandler, Asa C. (1916). "A study of the structure of feathers, with reference to their taxonomic significance". University of California Publications in Zoology. 13 (1): 243–446.CS1 maint: ref=harv (link)
- McGraw, K. J. 2005. Polly want a pigment? Cracking the chemical code to red coloration in parrots. Australian Birdkeeper Magazine 18:608–611.
- DeMeo, Antonia M. Access to Eagles and Eagle Parts: Environmental Protection v. Native American Free Exercise of Religion (1995)
- Electronic Code of Federal Regulations (e-CFR), Title 50: Wildlife and Fisheries PART 22—EAGLE PERMITS
- U.S. v. Thirty Eight Golden Eagles (1986)
- Mechanical structure of feathers
- Documentary on the evolution of feathers
- Lecture notes on the avian integument
- U.S. National Fish and Wildlife Forensics Laboratory's Feather Atlas
- Federn.org