Atom cluster
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term microcluster may be used for ensembles with up to couple dozen atoms.

Clusters with a definite number and type of atoms in a specific arrangement are often considered a specific chemical compound and are studied as such. For example, fullerene is a cluster of 60 carbon atoms arranged as the vertices of a truncated icosahedron, and decaborane is a cluster of 10 boron atoms forming an incomplete icosahedron, surrounded by 14 hydrogen atoms.
The term is most commonly used for ensembles consisting of several atoms of the same element, or of a few different elements, bonded in a three-dimensional arrangement. Transition metals and main group elements form especially robust clusters.[1] Indeed, in some contexts, the term may refer specifically to a metal cluster, whose core atoms are metals and contains at least one metallic bond.[2] In this case, the qualifier polynuclear specifies a cluster with more than one metal atom, and heteronuclear specifies a cluster with at least two different metal elements. Naked metal clusters have only metal atoms, as opposed to clusters with outer shell of other elements. The latter may be functional groups such as cyanide or methyl, covalently bonded to the core atoms; or many be ligands attached by coordination bonds, such as carbon monoxide, halides, isocyanides, alkenes, and hydrides.
However, the terms is also used for ensembles that contain no metals (such as the boranes and carboranes) and whose core atoms are held together by covalent or ionic bonds. It is also used for ensembles of atoms or molecules held together by Van der Waals or hydrogen bonds, as in water clusters.
Clusters may play an important role in phase transitions such as precipitation from solutions, condensation and evaporation of liquids and solids, freezing and melting, and adsorbtion to other materials.
History
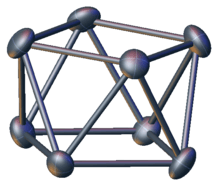
Atom cluster compounds, including metal clusters, have been unwittingly used by humans since antiquity. The oldest artificially produced metal cluster may be calomel Hg
2Cl
2, which was known in India already in the 12th century.
The elucidation of the structure of cluster compounds only became possible in the 20th century. For instance, the existence of a mercury to mercury bond in calomel was established in the early 1900s. These advances were made possible by the development of reliable structural analysis tools, such as single-crystal X-ray diffraction.
The term "cluster" was used by F.A. Cotton in the early 1960s to refer specifically to compounds containing metal–metal bonds.
Carbon clusters were first detected by Eric A. Rohlfing, Donald M. Cox, and Andrew Kaldor in 1984, in experiments where graphite was vaporized by laser and the vapor was quenched by a helium atmosphere. Analysis of the condensed products with a mass spectrometer revealed a preponderance of molecules with certain "magic numbers".[4] In 1985 their work was repeated by Harold Kroto, James R. Heath, Sean O'Brien, Robert Curl, and Richard Smalley, who proposed the truncated icosahedron structure for the prominent C60 molecule, and proposed the name "buckminsterfullerene" for it.[5]
Structure and stability
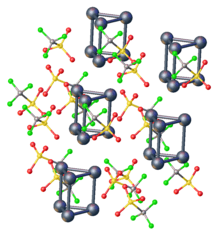
The physical and chemical properties of atom clusters are very different from those of bulk solid with the same composition. The difference is due to the fact that a large fraction of their component atoms is found at their surface. For cluster cores with fewer than a couple dozen component atoms or molecules, the stable configurations usually have most or all atoms adjacent to the core's surface, and thus only partially bound to other core elements.
A gradual transition occurs between the properties of the molecular species and those of the corresponding bulk mix with increasing number N of atoms in the core, since he fraction of atoms adjacent to its surface will scale approximately as N−1/3. If N is 105, when the cluster can be considered a nanoparticle, only about 10% of the atoms in the core will be exposed at its surface. That is still significant percentage, which is part of the reason why the properties of nanoparticles are still significantly different from those of the bulk substance.
Transition metal clusters are frequently composed of refractory metal atoms. In general metal centers with extended d-orbitals form stable clusters because of favorable overlap of valence orbitals. Thus, metals with a low oxidation state for the later metals and mid-oxidation states for the early metals tend to form stable clusters. Polynuclear metal carbonyls are generally found in late transition metals with low formal oxidation states. The polyhedral skeletal electron pair theory or Wade's electron counting rules predict trends in the stability and structures of many metal clusters. Jemmis mno rules have provided additional insight into the relative stability of metal clusters.
Gas-phase clusters and fullerenes
Unstable clusters can also be observed in the gas-phase by means of mass spectrometry, even though they may be thermodynamically unstable and aggregate easily upon condensation. Such naked clusters, i.e. those that are not stabilized by ligands, are often produced by laser induced evaporation - or ablation - of a bulk metal or metal-containing compound. Typically, this approach produces a broad distribution of size distributions. Their electronic structures can be interrogated by techniques such as photoelectron spectroscopy, while infrared multiphoton dissociation spectroscopy is more probing the clusters geometry.[7] Their properties (Reactivity, Ionization potential, HOMO–LUMO-gap) often show a pronounced size dependence. Examples of such clusters are certain aluminium clusters as superatoms and certain gold clusters. Certain metal clusters are considered to exhibit metal aromaticity. In some cases, the results of laser ablation experiments are translated to isolated compounds, and the premier cases are the clusters of carbon called the fullerenes, notably clusters with the formula C60, C70, and C84. The fullerene sphere can be filled with small molecules, forming Endohedral fullerenes.
Major families of cluster compounds
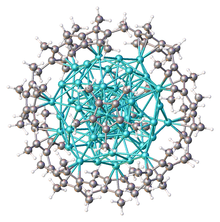
There is an infinite variety of compounds whose molecules are atom clusters or have such cluster at their core. Below are some classes that have received substantial attention from researchers.
Metallocarbohedrynes
Metallocarbohedrynes (or met-car for short) area family of clusters with molecular formula M
8C
12, where M is a transition metal such as titanium, vanadium, zirconium, niobium, hafnium, molybdenum, chromium, or iron. They can be generated by vaporizing the desired metal with a laser, in an atmosphere containing the suitable hydrocarbon. They have been also detected, at a concentration of 1% or less, in the soot generated by an electric arc between two Ti-C electrodes. They feature metals atoms at the corners of a cube, but with the carbon atoms pushed inwards so as to be nearly coplanar with the faces of that cube.
Zintl clusters
Zintl compounds feature naked anionic clusters that are generated by reduction of heavy main group p elements, mostly metals or semimetals, with alkali metals, often as a solution in anhydrous liquid ammonia or ethylenediamine.[9] Examples of Zintl anions are [Bi3]3−, [Sn9]4−, [Pb9]4−, and [Sb7]3−.[10] Although these species are called "naked clusters," they are usually strongly associated with alkali metal cations. Some examples have been isolated using cryptate complexes of the alkali metal cation, e.g., [Pb10]2− anion, which features a capped square antiprismatic shape.[11] According to Wade's rules (2n+2) the number of cluster electrons is 22 and therefore a closo cluster. The compound is prepared from oxidation of K4Pb9 [12] by Au+ in PPh3AuCl (by reaction of tetrachloroauric acid and triphenylphosphine) in ethylene diamine with 2.2.2-crypt. This type of cluster was already known as is the endohedral Ni@Pb102− (the cage contains one nickel atom). The icosahedral tin cluster Sn122− or stannaspherene anion is another closed shell structure observed (but not isolated) with photoelectron spectroscopy.[13][14] With an internal diameter of 6.1 Ångstrom, it is of comparable size to fullerene and should be capable of containing small atoms in the same manner as endohedral fullerenes, and indeed exists a Sn12 cluster that contains an Ir atom: [Ir@Sn12]3−.[15]
See also
- Cluster (physics)
- Water molecules form clusters as well: see water clusters
- Metallaprism
- Paolo Chini
- Metal carbonyl cluster
Further reading (reviews)
- Schnöckel, Hansgeorg (2010). "Structures and Properties of Metalloid al and Ga Clusters Open Our Eyes to the Diversity and Complexity of Fundamental Chemical and Physical Processes during Formation and Dissolution of Metals". Chemical Reviews. 110 (7): 4125–4163. doi:10.1021/cr900375g. PMID 20540559.
- Yano, Junko; Yachandra, Vittal (2014). "Mn4Ca Cluster in Photosynthesis: Where and How Water is Oxidized to Dioxygen". Chemical Reviews. 114 (8): 4175–4205. doi:10.1021/cr4004874. PMID 24684576.
- Dermota, T. E.; Zhong, Q.; Castleman, A. W. (2004). "Ultrafast Dynamics in Cluster Systems". Chemical Reviews. 104 (4): 1861–1886. doi:10.1021/cr020665e. PMID 15080714.
- Niedner-Schatteburg, Gereon; Bondybey, Vladimir E. (2000). "FT-ICR Studies of Solvation Effects in Ionic Water Cluster Reactions". Chemical Reviews. 100 (11): 4059–4086. doi:10.1021/cr990065o. PMID 11749340.
- Gabriel, Jean-Christophe P.; Boubekeur, Kamal; Uriel, Santiago; Batail, Patrick (2001). "Chemistry of Hexanuclear Rhenium Chalcohalide Clusters". Chemical Reviews. 101 (7): 2037–2066. doi:10.1021/cr980058k. PMID 11710240.
- Rohmer, Marie-Madeleine; Bénard, Marc; Poblet, Josep-M. (2000). "Structure, Reactivity, and Growth Pathways of Metallocarbohedrenes M8C12and Transition Metal/Carbon Clusters and Nanocrystals: A Challenge to Computational Chemistry". Chemical Reviews. 100 (2): 495–542. doi:10.1021/cr9803885. PMID 11749244.
- Muetterties, E. L.; Rhodin, T. N.; Band, Elliot.; Brucker, C. F.; Pretzer, W. R. (1979). "Clusters and Surfaces". Chemical Reviews. 79 (2): 91–137. doi:10.1021/cr60318a001.
References
- Inorganic Chemistry Huheey, JE, 3rd ed. Harper and Row, New York
- Mingos, D. M. P.; Wales, D. J. (1990). Introduction to cluster chemistry. Englewood Cliffs, N.J: Prentice Hall. ISBN 0134743059.
- Lindsjö, Andreas Fischer, Martin; Kloo, Lars (2005-02-01). "Improvements of and Insights into the Isolation of Bismuth Polycations from Benzene Solution – Single-Crystal Structure Determinations of Bi8[GaCl4]2 and Bi5[GaCl4]3". European Journal of Inorganic Chemistry. 2005 (4): 670–675. doi:10.1002/ejic.200400466. ISSN 1099-0682.
- Rohlfing, Eric A; Cox, D. M; Kaldor, A (1984). "Production and characterization of supersonic carbon cluster beams". Journal of Chemical Physics. 81 (7): 3322. Bibcode:1984JChPh..81.3322R. doi:10.1063/1.447994.
- Kroto, H. W.; Heath, J. R.; O'Brien, S. C.; Curl, R. F.; Smalley, R. E. (1985). "C60: Buckminsterfullerene". Nature. 318 (6042): 162–163. Bibcode:1985Natur.318..162K. doi:10.1038/318162a0.
- Schulz, Christopher; Daniels, Jörg; Bredow, Thomas; Beck, Johannes (2016). "The Electrochemical Synthesis of Polycationic Clusters". Angewandte Chemie International Edition. 55 (3): 1173–1177. doi:10.1002/anie.201507644. PMID 26632775.
- Fielicke A, Kirilyuk A, Ratsch A, Behler J, Scheffler M, von Helden G, Meijer G (2004). "Structure determination of isolated metal clusters via far-infrared spectroscopy" (PDF). Phys. Rev. Lett. 93 (2): 023401. Bibcode:2004PhRvL..93b3401F. doi:10.1103/PhysRevLett.93.023401. PMID 15323913.
- Vollet, Jean; Hartig, Jens R.; Schnöckel, Hansgeorg (2004). "Al50C120H180: A Pseudofullerene Shell of 60 Carbon Atoms and 60 Methyl Groups Protecting a Cluster Core of 50 Aluminum Atoms". Angewandte Chemie International Edition. 43 (24): 3186–3189. doi:10.1002/anie.200453754. PMID 15199573.
- S. Scharfe; F. Kraus; S. Stegmaier; A. Schier; T. F. Fässler (2011). "Homoatomic Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements". Angewandte Chemie International Edition. 50: 3630–3670. doi:10.1002/anie.201001630.
- Zintl Ions: Principles and Recent Developments, Book Series: Structure and Bonding. T. F. Fässler (Ed.), Volume 140, Springer, Heidelberg, 2011 doi:10.1007/978-3-642-21181-2
- A. Spiekermann; S. D. Hoffmann; T. F. Fässler (2006). "The Zintl Ion [Pb10]2−: A Rare Example of a Homoatomic closo Cluster". Angewandte Chemie International Edition. 45 (21): 3459–3462. doi:10.1002/anie.200503916. PMID 16622888.
- itself made by heating elemental potassium and lead at 350°C
- Tin particles are generated as K+Sn122− by laser evaporation from solid tin containing 15% potassium and isolated by mass spectrometer before analysis
- Li-Feng Cui; Xin Huang; Lei-Ming Wang; Dmitry Yu. Zubarev; Alexander I. Boldyrev; Jun Li; Lai-Sheng Wang (2006). "Sn122−: Stannaspherene". J. Am. Chem. Soc. 128 (26): 8390–8391. doi:10.1021/ja062052f. PMID 16802791.
- J.-Q. Wang; S. Stegmaier; B. Wahl; T. F. Fässler (2010). "Step by Step Synthesis of the Endohedral Stannaspherene [Ir@Sn12]3− via the Capped Cluster Anion [Sn9Ir(COD)]3−". Chem. Eur. J. 16: 3532–3552. doi:10.1002/chem.200902815.
External links
- http://cluster-science.net - scientific community portal for clusters, fullerenes, nanotubes, nanostructures, and similar small systems