Aldehyde
An aldehyde /ˈældɪhaɪd/ is a compound containing a functional group with the structure −CHO, consisting of a carbonyl center (a carbon double-bonded to oxygen) with the carbon atom also bonded to hydrogen and to a R group,[1] which is any generic alkyl or side chain. The group—without R—is the aldehyde group, also known as the formyl group. Aldehydes are common in organic chemistry, and many fragrances are or contain aldehydes.
Structure and bonding
Aldehydes feature an sp2-hybridized, planar carbon center that is connected by a double bond to oxygen and a single bond to hydrogen. The C–H bond is not ordinarily acidic. Because of resonance stabilization of the conjugate base, an α-hydrogen in an aldehyde (not shown in the picture above) is far more acidic, with a pKa near 17, compared to the acidity of a typical alkane (pKa about 50).[2] This acidification is attributed to (i) the electron-withdrawing quality of the formyl center and (ii) the fact that the conjugate base, an enolate anion, delocalizes its negative charge. Related to (i), the aldehyde group is somewhat polar. The formyl proton itself does not readily undergo deprotonation. The anionic species formally derived from deprotonation of an aldehyde proton, known as an acyl anion, is highly unstable and must be kept at low temperatures. In fact, with the exception of certain hindered dialkylformamides, the synthesis of acyl anions by direct deprotonation is not a feasible route, since the deprotonated species will almost immediately add to the highly reactive carbonyl of the starting material to form an acyloin compound. For this reason, the acidity of the formyl proton is difficult to measure. In the case of HCONiPr2, the acidity of the formyl group was found to be very close to that of diisopropylamine (pKa ~ 36).[3] The gas-phase acidity of formaldehyde was found to be 1,640 kJ/mol (393 kcal/mol), making it more acidic than hydrogen (1,700 kJ/mol, 400 kcal/mol) and ammonia (1,680 kJ/mol, 402 kcal/mol), but less acidic than water (1,600 kJ/mol, 390 kcal/mol) in the gas phase.[4]
Aldehydes (except those without an alpha carbon, or without protons on the alpha carbon, such as formaldehyde and benzaldehyde) can exist in either the keto or the enol tautomer. Keto–enol tautomerism is catalyzed by either acid or base. Usually the enol is the minority tautomer, but it is more reactive.
At around 360 kJ/mol (85 kcal/mol), the formyl C–H bond is weaker than that of a typical bond between hydrogen and an sp2-hybridized carbon. Thus aldehydes are prone to undergo hydrogen-atom abstraction in the presence of free radicals, a fact accounts for the ease with which aldehydes undergo autoxidation.
Nomenclature
IUPAC names for aldehydes
The common names for aldehydes do not strictly follow official guidelines, such as those recommended by IUPAC, but these rules are useful. IUPAC prescribes the following nomenclature for aldehydes:[5][6][7]
- Acyclic aliphatic aldehydes are named as derivatives of the longest carbon chain containing the aldehyde group. Thus, HCHO is named as a derivative of methane, and CH3CH2CH2CHO is named as a derivative of butane. The name is formed by changing the suffix -e of the parent alkane to -al, so that HCHO is named methanal, and CH3CH2CH2CHO is named butanal.
- In other cases, such as when a -CHO group is attached to a ring, the suffix -carbaldehyde may be used. Thus, C6H11CHO is known as cyclohexanecarbaldehyde. If the presence of another functional group demands the use of a suffix, the aldehyde group is named with the prefix formyl-. This prefix is preferred to methanoyl-.
- If the compound is a natural product or a carboxylic acid, the prefix oxo- may be used to indicate which carbon atom is part of the aldehyde group; for example, CHOCH2COOH is named 3-oxopropanoic acid.
- If replacing the aldehyde group with a carboxyl group (−COOH) would yield a carboxylic acid with a trivial name, the aldehyde may be named by replacing the suffix -ic acid or -oic acid in this trivial name by -aldehyde.
Etymology
The word aldehyde was coined by Justus von Liebig as a contraction of the Latin alcohol dehydrogenatus (dehydrogenated alcohol).[8][9] In the past, aldehydes were sometimes named after the corresponding alcohols, for example, vinous aldehyde for acetaldehyde. (Vinous is from Latin vinum "wine", the traditional source of ethanol, cognate with vinyl.)
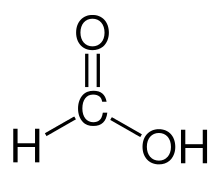
The term formyl group is derived from the Latin word formica "ant". This word can be recognized in the simplest aldehyde, formaldehyde (structure shown at top of article), and in the simplest carboxylic acid, formic acid (structure shown at right).
Physical properties and characterization
Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors.
Aldehydes, particularly arylaldehydes, degrade in air via the process of autoxidation. The acyl hydroperoxide is generated, which comproportionates with the starting material to generate two equivalents of the carboxylic acid. Old bottles of benzaldehyde, a liquid, will often accumulate a crusty solid on the bottle cap or even suspended in the bulk liquid. This material is benzoic acid, which can be removed by using a base wash followed by distillation.
The two aldehydes of greatest importance in industry, formaldehyde and acetaldehyde, have complicated behavior because of their tendency to oligomerize or polymerize. Formaldehyde in particular is sold as the polymer paraformaldehyde ((CH2O)n, typically n = 50 to 100) as well as the trimer 1,3,5-trioxane (metaformaldehyde). In addition to the inconveniently low-boiling monomer (b.p. 21 °C), acetaldehyde is available as the trimer paraldehyde (a sedative and anticonvulsant) and tetramer metaldehyde (a slug and snail poison). In general, higher aliphatic aldehydes will accumulate a substantial amount of oligomer (mostly trimer) upon long-term storage and must be freshly distilled when a reaction calls for the monomeric starting material. They also tend to hydrate, forming the geminal diol. Formaldehyde is often sold as the aqueous solution, formalin, which is mostly 1,1-methanediol, with a small amount of methanol added as stabilizer. The oligomers/polymers and the hydrates exist in equilibrium with the parent aldehyde, and for some synthetic procedures, they can serve as substitutes for the anhydrous monomer.
Aldehydes are readily identified by spectroscopic methods. Using IR spectroscopy, they display a strong νCO band near 1700 cm−1. In their 1H NMR spectra, the formyl hydrogen center absorbs near δH 9.5 to 10, which is a distinctive part of the spectrum. This signal shows the characteristic coupling to any protons on the α carbon with a small coupling constant typically less than 3.0 Hz. The 13C NMR spectra of aldehydes and ketones gives a suppressed (weak) but distinctive signal at δC 190 to 205.
Applications and occurrence
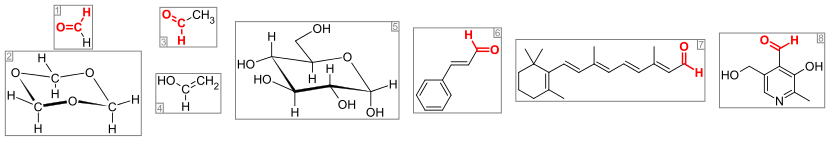
Important aldehydes and related compounds. The aldehyde group (or formyl group) is colored red. From the left: (1) formaldehyde and (2) its trimer 1,3,5-trioxane, (3) acetaldehyde and (4) its enol vinyl alcohol, (5) glucose (pyranose form as α-D-glucopyranose), (6) the flavorant cinnamaldehyde, (7) the visual pigment retinal, and (8) the vitamin pyridoxal.
Naturally occurring aldehydes
Traces of many aldehydes are found in essential oils and often contribute to their favorable odors, e.g. cinnamaldehyde, cilantro, and vanillin. Possibly because of the high reactivity of the formyl group, aldehydes are not common in several of the natural building blocks: amino acids, nucleic acids, lipids. Most sugars, however, are derivatives of aldehydes. These aldoses exist as hemiacetals, a sort of masked form of the parent aldehyde. For example, in aqueous solution only a tiny fraction of glucose exists as the aldehyde.
Synthesis
There are several methods for preparing aldehydes,[10] but the dominant technology is hydroformylation.[11] Illustrative is the generation of butyraldehyde by hydroformylation of propene:
- H2 + CO + CH3CH=CH2 → CH3CH2CH2CHO
Oxidative routes
Aldehydes are commonly generated by alcohol oxidation. In industry, formaldehyde is produced on a large scale by oxidation of methanol.[12] Oxygen is the reagent of choice, being "green" and cheap. In the laboratory, more specialized oxidizing agents are used, but chromium(VI) reagents are popular. Oxidation can be achieved by heating the alcohol with an acidified solution of potassium dichromate. In this case, excess dichromate will further oxidize the aldehyde to a carboxylic acid, so either the aldehyde is distilled out as it forms (if volatile) or milder reagents such as PCC are used.[13]
- [O] + CH3(CH2)9OH → CH3(CH2)8CHO + H2O
Oxidation of primary alcohols to form aldehydes can be achieved under milder, chromium-free conditions by employing methods or reagents such as IBX acid, Dess–Martin periodinane, Swern oxidation, TEMPO, or the Oppenauer oxidation.
Another oxidation route significant in industry is the Wacker process, whereby ethylene is oxidized to acetaldehyde in the presence of copper and palladium catalysts (acetaldehyde is also produced on a large scale by the hydration of acetylene).
On the laboratory scale, α-hydroxy acids are used as precursors to prepare aldehydes via oxidative cleavage.[14][15]
Specialty methods
| Reaction name | Substrate | Comment |
|---|---|---|
| Ozonolysis | Alkenes | Ozonolysis of non-fully-substituted alkenes yield aldehydes upon a reductive work-up. |
| Organic reduction | Esters | Reduction of an ester with diisobutylaluminium hydride (DIBAL-H) or sodium aluminium hydride. |
| Rosenmund reaction | Acyl chlorides | Acyl chlorides selectively reduced to aldehydes. Lithium tri-t-butoxyaluminium hydride (LiAlH(OtBu)3) is an effective reagent. |
| Wittig reaction | Ketones | A modified Wittig reaction using methoxymethylenetriphenylphosphine as a reagent. |
| Formylation reactions | Nucleophilic arenes | Various reactions, for example the Vilsmeier-Haack reaction. |
| Nef reaction | Nitro compounds | The acid hydrolysis of a primary nitro compound to form an aldehyde. |
| Kornblum oxidation | Haloalkanes | The oxidation of primary halide with dimethyl sulfoxide to form an aldehyde. |
| Zincke reaction | Pyridines | Zincke aldehydes formed in a reaction variation. |
| Stephen aldehyde synthesis | Nitriles | Hydrolysis of an iminium salt generated by tin(II) chloride and HCl to form an aldehyde. |
| Geminal halide hydrolysis | Geminal dihalides | Hydrolysis of primary geminal dihalides to yield aldehydes. |
| Meyers synthesis | Oxazines | Hemiaminal oxazine hydrolysis with water and oxalic acid to yield an aldehyde. |
| Hofmann rearrangement variation[16][17] | unsaturated or α-hydroxy amides | Aldehydes via the hydrolysis of an intermediate carbamate. |
| McFadyen-Stevens reaction | Hydrazides | Base-catalyzed thermal decomposition of acylsulfonylhydrazides. |
| Biotransformation | Alkenes | Lyophilized cell cultures of Trametes hirsuta in the presence of oxygen.[18] |
Common reactions
Aldehydes are highly reactive and participate in many reactions.[10] From the industrial perspective, important reactions are (a) condensations, e.g. to prepare plasticizers and polyols, and (b) reduction to produce alcohols, especially "oxo-alcohols". From the biological perspective, the key reactions involve addition of nucleophiles to the formyl carbon in the formation of imines (oxidative deamination) and hemiacetals (structures of aldose sugars).[10]
Reduction
The formyl group can be readily reduced to a primary alcohol (−CH2OH). Typically this conversion is accomplished by catalytic hydrogenation either directly or by transfer hydrogenation. Stoichiometric reductions are also popular, as can be effected with sodium borohydride.
Oxidation
The formyl group readily oxidizes to the corresponding carboxyl group (−COOH). The preferred oxidant in industry is oxygen or air. In the laboratory, popular oxidizing agents include potassium permanganate, nitric acid, chromium(VI) oxide, and chromic acid. The combination of manganese dioxide, cyanide, acetic acid and methanol will convert the aldehyde to a methyl ester.[19]
Another oxidation reaction is the basis of the silver-mirror test. In this test, an aldehyde is treated with Tollens' reagent, which is prepared by adding a drop of sodium hydroxide solution into silver nitrate solution to give a precipitate of silver(I) oxide, and then adding just enough dilute ammonia solution to redissolve the precipitate in aqueous ammonia to produce [Ag(NH3)2]+ complex. This reagent converts aldehydes to carboxylic acids without attacking carbon–carbon double bonds. The name silver-mirror test arises because this reaction produces a precipitate of silver, whose presence can be used to test for the presence of an aldehyde.
A further oxidation reaction involves Fehling's reagent as a test. The Cu2+ complex ions are reduced to a red-brick-coloured Cu2O precipitate.
If the aldehyde cannot form an enolate (e.g., benzaldehyde), addition of strong base induces the Cannizzaro reaction. This reaction results in disproportionation, producing a mixture of alcohol and carboxylic acid.
Nucleophilic addition reactions
Nucleophiles add readily to the carbonyl group. In the product, the carbonyl carbon becomes sp3-hybridized, being bonded to the nucleophile, and the oxygen center becomes protonated:
- RCHO + Nu− → RCH(Nu)O−
- RCH(Nu)O− + H+ → RCH(Nu)OH
In many cases, a water molecule is removed after the addition takes place; in this case, the reaction is classed as an addition–elimination or addition–condensation reaction. There are many variations of nucleophilic addition reactions.
Oxygen nucleophiles
In the acetalisation reaction, under acidic or basic conditions, an alcohol adds to the carbonyl group and a proton is transferred to form a hemiacetal. Under acidic conditions, the hemiacetal and the alcohol can further react to form an acetal and water. Simple hemiacetals are usually unstable, although cyclic ones such as glucose can be stable. Acetals are stable, but revert to the aldehyde in the presence of acid. Aldehydes can react with water to form hydrates, R−CH(OH)2. These diols are stable when strong electron withdrawing groups are present, as in chloral hydrate. The mechanism of formation is identical to hemiacetal formation.
Nitrogen nucleophiles
In alkylimino-de-oxo-bisubstitution, a primary or secondary amine adds to the carbonyl group and a proton is transferred from the nitrogen to the oxygen atom to create a carbinolamine. In the case of a primary amine, a water molecule can be eliminated from the carbinolamine intermediate to yield an imine or its trimer, a hexahydrotriazine This reaction is catalyzed by acid. Hydroxylamine (NH2OH) can also add to the carbonyl group. After the elimination of water, this results in an oxime. An ammonia derivative of the form H2NNR2 such as hydrazine (H2NNH2) or 2,4-dinitrophenylhydrazine can also be the nucleophile and after the elimination of water, resulting in the formation of a hydrazone, which are usually orange crystalline solids. This reaction forms the basis of a test for aldehydes and ketones.[20]
Carbon nucleophiles
The cyano group in HCN can add to the carbonyl group to form cyanohydrins, R−CH(OH)CN. In this reaction the CN− ion is the nucleophile that attacks the partially positive carbon atom of the carbonyl group. The mechanism involves a pair of electrons from the carbonyl-group double bond transferring to the oxygen atom, leaving it single-bonded to carbon and giving the oxygen atom a negative charge. This intermediate ion rapidly reacts with H+, such as from the HCN molecule, to form the alcohol group of the cyanohydrin.
Organometallic compounds, such as organolithium reagents, Grignard reagents, or acetylides, undergo nucleophilic addition reactions, yielding a substituted alcohol group. Related reactions include organostannane additions, Barbier reactions, and the Nozaki–Hiyama–Kishi reaction.
In the aldol reaction, the metal enolates of ketones, esters, amides, and carboxylic acids add to aldehydes to form β-hydroxycarbonyl compounds (aldols). Acid or base-catalyzed dehydration then leads to α,β-unsaturated carbonyl compounds. The combination of these two steps is known as the aldol condensation.
The Prins reaction occurs when a nucleophilic alkene or alkyne reacts with an aldehyde as electrophile. The product of the Prins reaction varies with reaction conditions and substrates employed.
Bisulfite reaction
Aldehydes characteristically form "addition compounds" with sodium bisulfite:
- RCHO + HSO−
3 → RCH(OH)SO−
3
This reaction is used as a test for aldehydes.[20]
More complex reactions
| Reaction name | Product | Comment |
|---|---|---|
| Wolff–Kishner reduction | Alkane | If an aldehyde is converted to a simple hydrazone (RCH=NHNH2) and this is heated with a base such as KOH, the terminal carbon is fully reduced to a methyl group. The Wolff–Kishner reaction may be performed as a one-pot reaction, giving the overall conversion RCH=O → RCH3. |
| Pinacol coupling reaction | Diol | With reducing agents such as magnesium |
| Wittig reaction | Alkene | Reagent: an ylide |
| Takai reaction | Alkene | Diorganochromium reagent |
| Corey–Fuchs reactions | Alkyne | Phosphine-dibromomethylene reagent |
| Ohira–Bestmann reaction | Alkyne | Reagent: dimethyl (diazomethyl)phosphonate |
| Johnson–Corey–Chaykovsky reaction | Epoxide | Reagent: a sulfonium ylide |
| Oxo-Diels–Alder reaction | Pyran | Aldehydes can, typically in the presence of suitable catalysts, serve as partners in cycloaddition reactions. The aldehyde serves as the dienophile component, giving a pyran or related compound. |
| Hydroacylation | Ketone | In hydroacylation an aldehyde is added over an unsaturated bond to form a ketone. |
| decarbonylation | Alkane | Catalysed by transition metals |
Dialdehydes
A dialdehyde is an organic chemical compound with two aldehyde groups. The nomenclature of dialdehydes have the ending -dial or sometimes -dialdehyde. Short aliphatic dialdehydes are sometimes named after the diacid from which they can be derived. An example is butanedial, which is also called succinaldehyde (from succinic acid).
Biochemistry
Some aldehydes are substrates for aldehyde dehydrogenase enzymes which metabolize aldehydes in the body. There are toxicities associated with some aldehydes that are related to neurodegenerative disease, heart disease, and some types of cancer.[21]
Examples of aldehydes
- Formaldehyde (methanal)
- Acetaldehyde (ethanal)
- Propionaldehyde (propanal)
- Butyraldehyde (butanal)
- Benzaldehyde (phenylmethanal)
- Cinnamaldehyde
- Vanillin
- Tolualdehyde
- Furfural
- Retinaldehyde
Examples of dialdehydes
Uses
Of all aldehydes, formaldehyde is produced on the largest scale, about 6000000 tons per year. It is mainly used in the production of resins when combined with urea, melamine, and phenol (e.g., Bakelite). It is a precursor to methylene diphenyl diisocyanate ("MDI"), a precursor to polyurethanes.[12] The second main aldehyde is butyraldehyde, of which about 2500000 tons per year are prepared by hydroformylation. It is the principal precursor to 2-ethylhexanol, which is used as a plasticizer.[22] Acetaldehyde once was a dominating product, but production levels have declined to less than 1000000 tons per year because it mainly served as a precursor to acetic acid, which is now prepared by carbonylation of methanol. Many other aldehydes find commercial applications, often as precursors to alcohols, the so-called oxo alcohols, which are used in detergents. Some aldehydes are produced only on a small scale (less than 1000 tons per year) and are used as ingredients in flavours and perfumes such as Chanel No. 5. These include cinnamaldehyde and its derivatives, citral, and lilial.
See also
References
- IUPAC Gold Book, aldehydes.
- Chemistry of Enols and Enolates – Acidity of alpha-hydrogens.
- Fraser, Robert R.; Hubert, Patrick R. (January 1974). "Direct Formation of the Carbonyl Anion of Diisopropyl Formamide". Canadian Journal of Chemistry. 52 (1): 185–187. doi:10.1139/v74-029. ISSN 0008-4042.
- Ervin, Kent M.; DeTuri, Vincent F. (October 2002). "Anchoring the Gas-Phase Acidity Scale". The Journal of Physical Chemistry A. 106 (42): 9947–9956. doi:10.1021/jp020594n. ISSN 1089-5639.
- Short Summary of IUPAC Nomenclature of Organic Compounds Archived 2006-09-01 at the Wayback Machine, web page, University of Wisconsin Colleges, accessed on line August 4, 2007.
- §R-5.6.1, Aldehydes, thioaldehydes, and their analogues, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
- §R-5.7.1, Carboxylic acids, A Guide to IUPAC Nomenclature of Organic Compounds: recommendations 1993, IUPAC, Commission on Nomenclature of Organic Chemistry, Blackwell Scientific, 1993.
- Liebig, J. (1835) "Sur les produits de l'oxidation de l'alcool" (On the products of the oxidation of alcohol), Annales de Chimie et de Physique, 59 : 289–327. From page 290: "Je le décrirai dans ce mémoire sous le nom d'aldehyde ; ce nom est formé de alcool dehydrogenatus." (I will describe it in this memoir by the name of aldehyde; this name is formed from alcohol dehydrogenatus.)
- Crosland, Maurice P. (2004), Historical Studies in the Language of Chemistry, Courier Dover Publications, ISBN 9780486438023.
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- Bertleff, W.; Roeper, M. and Sava, X. (2003) "Carbonylation" in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH: Weinheim. doi:10.1002/14356007.a05_217.pub2
- Reuss, G.; Disteldorf, W.; Gamer, A. O. and Hilt, A. (2005) "Formaldehyde" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_619.
- Ratcliffe, R. W. (1988). "Oxidation with the Chromium Trioxide-Pyridine Complex Prepared in situ: 1-Decanal". Organic Syntheses.; Collective Volume, 6, p. 373
- Ōeda, Haruomi (1934). "Oxidation of some α-hydroxy-acids with lead tetraacetate". Bulletin of the Chemical Society of Japan. 9 (1): 8–14. doi:10.1246/bcsj.9.8.
- Nwaukwa, Stephen; Keehn, Philip (1982). "Oxidative cleavage of α-diols, α-diones, α-hydroxy-ketones and α-hydroxy- and α-keto acids with calcium hypochlorite [Ca(OCl)2]". Tetrahedron Letters. 23 (31): 3135–3138. doi:10.1016/S0040-4039(00)88578-0.
- Weerman, R.A. (1913). "Einwirkung von Natriumhypochlorit auf Amide ungesättigter Säuren". Justus Liebigs Annalen der Chemie. 401 (1): 1–20. doi:10.1002/jlac.19134010102.
- Everett, Wallis; Lane, John (1946). The Hofmann Reaction. Organic Reactions. 3. pp. 267–306. doi:10.1002/0471264180.or003.07. ISBN 9780471005285.
- Sutton, Peter; Whittall, John (2012). Practical Methods for Biocatalysis and Biotransformations 2. Chichester, West Sussex: John Wiley & Sons, Ltd. pp. 199–202. ISBN 9781119991397.
- Corey, Elias J.; Gilman, Norman W.; Ganem, B. E. (1968). "New methods for the oxidation of aldehydes to carboxylic acids and esters". J. Am. Chem. Soc. 90 (20): 5616–5617. doi:10.1021/ja01022a059.
- Shriner, R. L.; Hermann, C. K. F.; Morrill, T. C.; Curtin, D. Y.; Fuson, R. C. (1997). The Systematic Identification of Organic Compounds. John Wiley & Sons. ISBN 978-0-471-59748-3.
- Chen, Che-Hong; Ferreira, Julio Cesar Batista; Gross, Eric R.; Rosen, Daria Mochly (1 January 2014). "Targeting Aldehyde Dehydrogenase 2: New Therapeutic Opportunities". Physiological Reviews. 94 (1): 1–34. doi:10.1152/physrev.00017.2013. PMC 3929114. PMID 24382882.
- Kohlpaintner, C.; Schulte, M.; Falbe, J.; Lappe, P. and Weber, J. (2008) "Aldehydes, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_321.pub2.
External links
| Look up aldehyde in Wiktionary, the free dictionary. |
| Wikiquote has quotations related to: Aldehyde |
- Aldehyde synthesis – Synthetic protocols from organic-reaction.com