Selone
In chemistry, a selone (also known as a selenoketone) is the structural analog of a ketone where selenium replaces oxygen. Selenium-77 is one of the isotopes of selenium that is stable and naturally occurring, so selenone-containing chemicals can be analyzed by nuclear magnetic resonance spectroscopy (NMR). Selones can be used as chiral derivatizing agents for 77Se-NMR.[1] Chiral oxazolidineselones can be used for stereoselective control of aldol reactions, analogous to the Evans aldol reaction that uses oxazolidinones, which allows 77Se-NMR to be used to determine the diastereomeric ratio of the aldol product.[2]
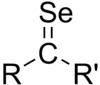
In constast to analogous structures with earlier chalcogens, selones greater steric and electronic stabilization.[3] Selenobenzophenone reversibly dimerizes. It is known to undergo cycloaddition with 1,3-dienes in a reaction similar to the Diels-Alder reaction.[4]
References
- J. Peng; J. D. Odom; R. B. Dunlap & L. A. Silks III (1994). "Use of a selone chiral derivatizing agent for the absolute configurational assignment of stereogenic center". Tetrahedron: Asymmetry. 5 (9): 1627–1630. doi:10.1016/0957-4166(94)80066-9.
- L. Silks; D. Kimball; D. Hatch; et al. (2009). "Chiral N-Acetyl Selone-Promoted Aldol Reactions" (PDF). Synthetic Communications. 39 (4): 641–653. doi:10.1080/00397910802419706.
- Okazaki, R.; Tokitoh, N. (2000). "Heavy ketones, the heavier element congeners of a ketone". Accounts of Chemical Research. 33 (9): 625–630. doi:10.1021/ar980073b. PMID 10995200.
- Erker, G.; Hock, R.; Krüger, C.; Werner, S.; Klärner, F. G.; Artschwager-Perl, U. (1990). "Synthesis and Cycloadditions of Monomeric Selenobenzophenone". Angewandte Chemie International Edition in English. 29 (9): 1067. doi:10.1002/anie.199010671.