Phosphonite
Phosphonites are organophosphorus compounds with the formula P(OR)2R. They are found in some pesticides and are used as ligands.[1]
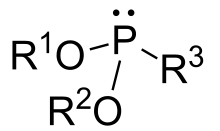
General ester of phosphonous acid
Preparation
Although they are derivatives of phosphonous acid (RP(OH)2),[2] they are not prepared from such precursors. Phosphonites are prepared by alcoholysis of organophosphinous chlorides. For example, treatment of dichlorophenylphosphine with methanol and base gives dimethyl phenylphosphonite:
- Cl2PPh + 2 CH3OH → (CH3O)2PPh + 2 HCl
Reactions
Oxidation of phosphonites gives phosphonates:
- 2 P(OR)2R + O2 → 2 OP(OR)2R
Phosphonites can function as ligands in homogeneous catalysis.[3]
gollark: (I don't think that "how big is a molecule of it" is really a valid question, or at least one you can work out that way, but I am not very sure)
gollark: <@474726021652807680> If you used that molar mass they have, you would be calculating the mass of a mole of it, which isn't a molecule.
gollark: What mass are you using? You said you wanted to know how big a molecule was or something?
gollark: In that case, put in a mass in grams, and the density in g/L, and you'll get a volume in litres.
gollark: If you get the density in, say, kg/m³, then the mass is in kg and volume is in m³.
References
- D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam 1995. ISBN 0-444-89307-5.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "phosphonous acids". doi:10.1351/goldbook.P04565
- T. V. (Babu) Rajanbabu “Phosphinite and Phosphonite Ligands” in Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis Paul C. J. Kamer and Piet W. N. M. van Leeuwen, Eds., John Wiley & Sons 2012. doi:10.1002/9781118299715.ch5
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.