Acrylate polymer
Acrylate polymers are a group of polymers noted for their transparency, resistance to breakage, and elasticity. They are also commonly known as acrylics or polyacrylates. Acrylate polymer is commonly used in cosmetics, such as nail polish, as an adhesive.[1]
Monomers
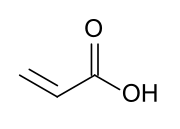
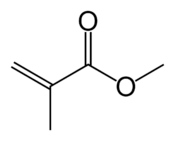
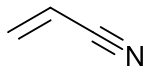
Acrylate monomers, used to form acrylate polymers, are based on the structure of acrylic acid,[2] which consists of a vinyl group and a carboxylic acid ester end or a nitrile.[3][4] Other typical acrylate monomers are derivatives of acrylic acid, such as methyl methacrylate in which one vinyl hydrogen and the carboxylic acid hydrogen are both replaced by methyl groups, and acrylonitrile in which the carboxylic acid group is replaced by the related nitrile group.
Other examples of acrylate monomers are:
- Methacrylates[5]
- Methyl acrylate
- Ethyl acrylate
- 2-Chloroethyl vinyl ether
- 2-Ethylhexyl acrylate
- Hydroxyethyl methacrylate
- Butyl acrylate
- Butyl methacrylate
- TMPTA
Acrylic elastomers
Acrylic elastomer is a general term for a type of synthetic rubber whose primary component is acrylic acid alkylester (ethyl or butyl ester).[6] Acrylic elastomer possesses characteristics of heat and oil resistance, with the ability to withstand temperatures of 170–180 ℃. It is used primarily for producing oil seals and packaging related to automobiles.
Acrylic elastomer can generally be characterized as one of two types. "Old" types include ACM (copolymer of acrylic acid ester and 2-chloroethyl vinyl ether) containing chlorine and ANM (copolymer of acrylic acid ester and acrylonitrile) without chloride. "New" types do not contain chlorine and are less prone to mold-related staining. Other than the slightly better water resistance of ANM there are no physical differences between the two types.
The material is less resistant in terms of cold weather with a saturation point of −15 ℃ for old types and −28 to −30 ℃ for new types. In terms of vulcanization, the standard method for the old type is amine vulcanization. To minimize permanent deformation, the old type requires curing for 24 hours at a temperature of 150 ℃. On the other hand, for the new type, the press curing time and follow-up vulcanization time are significantly reduced by combining metal soap and sulfur. It has no special characteristics. The rebound resilience and abrasion resistance of the new type are poor, and even its electrical characteristics are considerably poor compared with acrylonitrile-butadiene rubber and butyl rubber.
Uses
- Polyacrylate emulsion, water-borne coating, are used as binder for outdoor and indoor "latex" house paints.
- Acrylic paints as artist paints
- Acrylic fibre
- Sodium polyacrylate water-soluble thickeners, a polymer for the production of the Superabsorbent polymer (SAP) used in disposable diapers due to its high absorbency per unit mass
- Acrylic resin as pressure-sensitive adhesive
- "Super glue" is a formulation of cyanoacrylate.
- Polyacrylates are used in cosmetic products as rheology modifiers and film formers, and these are typically polymers of acrylic acid
Related polymers
- PVAc copolymer emulsion glue of vinyl acetate (VAM) and acrylic acid (VAA)
- Polyacrylamide copolymer used as flocculation agent in water treatment
- Polymethyl methacrylate, is the clear break-resistant sheeting sold as acrylic glass (or simply acrylic sheet) or under the trade name Plexiglas, Perspex, etc.
See also
- (Meth)acrylates
- Acrylic (disambiguation)
- Dishmaker
References
- Erich Penzel (2000). "Polyacrylates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_157. ISBN 3527306730.
- Mignon, Arn; Devisscher, Dries; Graulus, Geert-Jan; Stubbe, Birgit; Martins, José; Dubruel, Peter; De Belie, Nele; Van Vlierberghe, Sandra (2017-01-02). "Combinatory approach of methacrylated alginate and acid monomers for concrete applications". Carbohydrate Polymers. 155: 448–455. doi:10.1016/j.carbpol.2016.08.102. hdl:1942/22766. ISSN 0144-8617. PMID 27702534.
- Takashi Ohara; Takahisa Sato; Noboru Shimizu; et al. (2002). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_161.pub2. ISBN 3527306730.
- http://pslc.ws/macrog/acrylate.htm
- Manfred Stickler; Thoma Rhein (2000). "Polymethacrylates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_473. ISBN 3527306730.
- "Archived copy". Archived from the original on 2011-06-12. Retrieved 2010-05-26.CS1 maint: archived copy as title (link)