(Hydroxyethyl)methacrylate
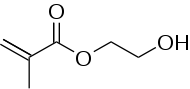
Hydroxyethylmethacrylate or HEMA is the organic compound with the formula H2C=C(CH3)CO2CH2CH2OH. It is a colorless viscous liquid that readily polymerizes. HEMA is a monomer that is used to make various polymers.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Hydroxyethyl 2-methylprop-2-enoate | |
| Other names
HEMA; hydroxyethylmethacrylate; glycol methacrylate; glycol monomethacrylate; hydroxyethyl methacrylate; ethylene glycol methacrylate; 2-(methacryloyloxy)ethanol | |
| Identifiers | |
3D model (JSmol) |
|
| 1071583 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.621 |
| EC Number |
|
| 936557 | |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H10O3 | |
| Molar mass | 130.143 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.07 g/cm3 |
| Melting point | −99 °C (−146 °F; 174 K)[1] |
| Boiling point | 213 °C (415 °F; 486 K)[1] |
| miscible | |
| log P | 0.50[2] |
| Vapor pressure | 0.08 hPa |
| Hazards | |
| Main hazards | Eye irritation |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H317, H319 |
| P261, P264, P272, P280, P302+352, P305+351+338, P321, P332+313, P333+313, P337+313, P362, P363, P501 | |
| Flash point | 97 °C (207 °F; 370 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
Polyhydroxyethylmethacrylate is hydrophobic; however, when the polymer is subjected to water it will swell due to the molecule's hydrophilic pendant group. Depending on the physical and chemical structure of the polymer, it is capable of absorbing from 10 to 600% water relative to the dry weight. Because of this property, it was one of the first materials to be successfully used in the manufacture of soft contact lenses[3]
When treated with polyisocyanates, poly(HEMA) makes a crosslinked polymer, an acrylic resin, that is a useful component in some paints.[4]
Use in 3D printing
HEMA lends itself well to applications in 3D printing as it cures quickly at room temperature when exposed to UV light in the presence of photoinitiators. It may be used as a monomeric matrix in which 40nm silica particles are suspended for 3D glass printing.[5] When combined with a suitable blowing agent such as BOC Anhydride it forms a foaming resin which expands when heated.[6]
References
- "GPS Safety Summary 2-Hydroxyethyl methacrylate (HEMA)". July 2013.
- "2-hydroxyethyl methacrylate_msds".
- Blasco, Joe; Kehoe, Vincent J-R; The professional make-up artist : motion pictures, television, print, theatre; ISBN 0-9771580-0-4; LCC# PN2068.B53 2005
- Stoye, D.; Funke, W.; Hoppe, L.; et al. (2006). "Paints and Coatings". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_359.pub2.
- Kotz (20 April 2017). "Three-dimensional printing of transparent fused silica glass". Nature. 544 (7650): 337–339. doi:10.1038/nature22061. PMID 28425999.
- Wirth, D. (8 April 2020). "Highly Expandable Foam for Lithographic 3D Printing". ACS Appl. Mater. Interfaces. 12 (16): 19033–19043. doi:10.1021/acsami.0c02683. PMID 32267677.