Acamptonectes
Acamptonectes (meaning "rigid swimmer") is an extinct genus of ophthalmosaurid ichthyosaur that lived between 134 and 132 million years ago during the Early Cretaceous. Known from fossil deposits in England and Germany, the genus currently contains the single species A. densus, which was first described in 2012. Its discovery was important in the research of ichthyosaurs, as being one of the first known ophthalmosaurines from the Early Cretaceous, it provided evidence that no mass extinction of ichthyosaurs occurred during the Jurasic-Cretaceous boundary as previously understood. Nevertheless, Acamptonectes remains one of only eight genera of ichthyosaurs known during the Cretaceous. In life, Acamptonectes was probably a generalist predator. Its teeth were slender and textured with longitudinal ridges, which were best adapted for impaling prey; as a result, Acamptonectes likely fed on soft, fleshier prey such as fish and squid. The ichthyosaur's body was rigid and compact, which was suited for swimming at high speeds in tuna-like locomotion.
| Acamptonectes | |
|---|---|
| Specimen SNHM1284-R in State Natural History Museum, Braunschweig | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | †Ichthyosauria |
| Family: | †Ophthalmosauridae |
| Subfamily: | †Ophthalmosaurinae |
| Genus: | †Acamptonectes Fischer et al. 2012 |
| Species: | †A. densus |
| Binomial name | |
| †Acamptonectes densus Fischer et al. 2012 | |
History of discovery

Over a series of weekends in 1958, four students and a technician from the geology department of Hull University collected an ichthyosaur specimen from the Speeton Clay Formation of Speeton in northern England. It was transferred to the Hunterian Museum of the University of Glasgow in 1991, when the geology department of Hull University was closed, where it was catalogued as specimen GLAHM 132855 (it was also known as the "Speeton Clay ichthyosaur"). It consists of a partial skeleton of an adult, including a fragmentary skull roof, a mandible, the axial skeleton, and the scapular girdle. Palaeontologist Robert M. Appleby described the specimen and assigned it to the genus Platypterygius as the species "P. speetoni" (which he considered primitive within that genus), in a monograph that remained unpublished at the time of his death in 2003. A second specimen of this ichthyosaur was found in 1985, also in the Speeton Clay, and is catalogued as NHMUK R11185 at the Natural History Museum, London. It consists of a partial rostrum and mandible, fragmentary ribs, and a complete right humerus.[3][4][5]
Palaeontologist Jeff Liston, who had recognised the significance of the Speeton Clay icthyosaur while working at the Hunterian Museum, and had been asked by Appleby's widow to help finish his unpublished monograph, approached ichthyosaur specialist Valentin Fischer about writing a description of the animal. Fischer examined the specimen in 2011, and realised it represented the same taxon as an ichthyosaur specimen from Cremlingen in northern Germany, which he had recently written a draft paper about with some colleagues. The German specimen was discovered in 2005, when private fossil collector Hans-Dieter Macht found some vertebrae in a construction area. Macht notified the director of the State Natural History Museum of Braunschweig, whereafter excavation began; the specimen was collected within three days, since construction work had to continue. It was prepared and mounted at the museum in 2005, where it is catalogued as SNHM1284-R. It consists of a partial skeleton of a subadult, including a fragmentary skull roof, a complete mandible, a partial axial skeleton, and a partial scapular girdle.[3][4] It was referred to as Platypterygius in a 2008 article.[6]
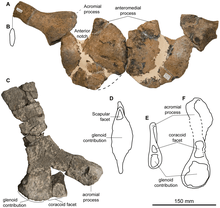
After determining that the Speeton Clay specimen was much larger than that from Cremlingen, Liston and Fischer decided to make it the holotype of the new species (since juvenile traits are often not found in adults), with the Cremlingen specimen as paratype, and the other Speeton Clay specimen as an additional paratype. A team of palaeontologists led by Fischer formally named the new genus and species Acamptonectes densus in 2012. The generic name is derived from the Greek words akamptos and nektes, which means rigid swimmer, and the specific name means compact, or tightly packed. In full, the binomial name refers to the robust, tightly fitting bones of the occiput, and the tightly interlocking centra ("bodies") of the cervical (neck) and dorsal (back) vertebrae.[3][4][7] The holotype, GLAHM 132855, was listed under the inaccurate specimen number GLAHM 132588 in the description.[5]
In 1909, German palaeontologist Ferdinand Broili named the species Ichthyosaurus brunsvicensis (tentatively placed in that genus) based on an ichthyosaur specimen from Hannover, Germany. It consisted of an incomplete basicranium and an incomplete interclavicle, but was destroyed during WW2. Palaeontologist Christopher McGowan considered it to belong to Platypterygius in 1972 and 2003, but Fischer and colleagues found it appropriate to assign it to cf. Acamptonectes (difficult to identify). They found it similar to Acamptonectes in several features, while also differing in some ways, and suggested it was a juvenile due to the size and shape of its basicranium. They considered I. brunsvicensis a nomen dubium due to being fragmentary, as well as not being available for study anymore.[3][8][9]
Multiple basioccipitals, stapes, and a basisphenoid from the Cambridge Greensand Formation of Cambridge, England, were also assigned to Acamptonectes sp. (of uncertain species within the genus) by Fischer and colleagues. Some of these elements are essentially identical to those of A. densus, while others differ in some details. The specimens are generally small, and their differences from A. densus may be related to the ages of the animals or evolutionary changes. Most of these specimens are housed at Sedgwick Museum of Earth Sciences, Cambridge, with a few at the Hunterian Museum and Natural History Museum.[3] In 2014, though, Fischer and colleagues found this identification disputable, since Acamptonectes was no longer the only known Cretaceous ophthalmosaurine ichthyosaur from Eurasia. They therefore listed the material from the Cambridge Greensand as belonging to indeterminate ophthalmosaurines, not identifiable below the subfamily level.[10]
Description
Acamptonectes was similar in morphology to the related but earlier ophthalmosaurids Ophthalmosaurus and Mollesaurus. Features of the humerus in specimen SNHM1284-R indicate immaturity, but it lacks the sandpaper-like texture present on the shaft of the humerus in juvenile ichthyosaurs, so is thought to be a subadult. The holotype and NHMUK R11185 are large ophthalmosaurids, and the former is thought to be an adult due to the full fusion of its bones, as seen in the closely fitting occiput bones, and the smooth texture of the humerus.[3] As an ichthyosaur, Acamptonectes had a long, thin snout, large eye sockets, and a tail fluke (the lower part of which was supported by vertebrae), and they were therefore superficially similar to dolphins. They had flippers instead of legs, and except for early species, had dorsal fins.[11][12] Although no evidence is specific to Acamptonectes, at least some ichthyosaurs may have been uniformly dark-colored in life, demonstrated by the discovery of high eumelanin contents in the preserved skin of an older ichthyosaur fossil.[13]
Skull
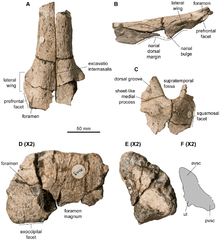
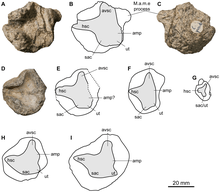
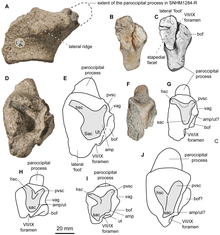
The snout was elongated and extremely slender – in front of the bony nostrils, it was only 45 mm wide in the holotype specimen. The snout also was only 0.044 times as deep as long, which is one of the lowest ratios found in ophthalmosaurids. Much of the snout was formed by the premaxillae, which formed the front portion of the upper jaw. The fossa praemaxillaris, a groove that ran parallel with the tooth row of the upper jaw, was deep and continuous, ending in a series of aligned foramina (depressions). Behind and above the premaxillae were the nasals, which are three-dimensionally preserved in the holotype, documenting the shape of the upper side of the snout. On its back part, the nasal featured a downward-extending bulge, similar to that seen in some related genera including Ophthalmosaurus. This bulge also gave rise to a short but robust wing-like extension that formed an overhang over the hind part of the bony nostril; this feature was also present in Ophthalmosaurus icenicus and Platypterygius australis. The edge of this overhang was roughened, indicating that this was probably the attachment site for a soft-tissue structure. The hind part of the skull roof is only incompletely known, including the hind part of the lacrimal (in front of the eye opening), the postfrontal (above and behind the eye opening), the parietal (at the rear of the skull roof), and parts of a supratemporal (which formed the rear corners of the skull roof). An forward-directed extension of the supratemporal formed the inner-rear edge of the supratemporal fenestra, an opening through the skull roof behind the eyes. The parietal, which would have formed the inner margin of the supratemporal fenestra, had a complex front margin that was interdigitating with either the frontal or postfrontal bones, which are not preserved in the known specimens.[3][14]:20–22
The quadrate bone, which connected to the lower jaw to form the jaw joint, was C-shaped in side view. Two probable hyoid bones (tongue bones) are preserved with specimen SNHM1284-R; these bones were rod-like, with one end spatula-shaped. The stapes (bone in the ear region that is used for hearing) had a shaft that was more slender than in any other ichthyosaur, and its head was large and square—these features are regarded an autapomorphy (unique feature) and serve to distinguish the genus from related genera. The basisphenoid, a bone of the lower part of the braincase, had a well-developed crest on its upper surface, which also is considered an autapomorphy. In other ichthyosaurs, the basisphenoid instead formed a wide plateau. At its front end, the basisphenoid was fused to the parasphenoid, and no suture (border between the two bones) can be seen. The supraoccipital at the upper rear of the braincase was only weakly arched and thus different from Platypterygius and Ophthalmosaurus natans, where it was U-shaped. Below the supraoccipital were the two exoccipitals, which formed the sides of the foramen magnum, the canal for the spinal cord. Further below, and forming the floor of the foramen magnum, was the basioccipital. The midline canal that formed this floor was bordered by ridges, giving a bilobed appearance when seen from above, which is regarded an autapomorphy of the genus. Below the foramen magnum, the basioccipital formed the occipital condyle to connect with the first vertebra of the neck, forming the head joint. The occipital condyle was well demarcated from the remainder of the bone by a constricted band, unlike in most other ophthalmosaurids. The condyle was rounded and had visible growth rings, as in related genera. The opisthotics, which sat at both sides of the basioccipital, had extensions pointing backwards and upwards, the paroccipital processes. These processes were elongated and slender in Acamptonectes and Ophthalmosaurus icenicus, but short and stout in other ophthalmosaurids.[3][14]
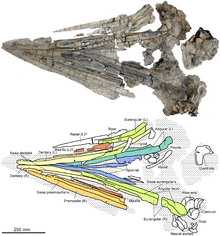
The dentary, the tooth-bearing bone of the lower jaw, was elongated, straight, and had a blunt front tip, contrasting with the downturned and beak-like tips of some platypterygiines. The splenial bones expanded hindwards in depth to form the lower border of the mandible and much of its midline surface. The fossa dentalis was equivalent to the groove that ran parallel with the upper tooth row, and similar in morphology. Two Acamptonectes specimens lacked the “3”-shaped upper surface of the angular bones otherwise typical of ophthalmosaurids, instead having a simple, flat groove bordered by two walls. But since the "3" shape is present in the holotype specimen, this feature may have been variable between individuals or growth stages. The articular bone in one specimen was stouter than in other ophthalmosaurids, nearly as thick as long. The roots of the teeth were striated at their bases, and some were roughly quadrangular, as in many other ophthalmosaurids, but not square-shaped as in Platypterygius. Some of the roots of SNHM1284-R had resorption pits, indicating it was still growing teeth. The size of the only known complete tooth crown was relatively small compared to other ophthalmosaurids, slender, and sharply pointed, similar to the hind teeth of Baptanodon. The basal two thirds of the crown had subtle, longitudinal ridges, and was covered in a coarse texture, finer than in Aegirosaurus and some Platypterygius secimens. The base of the crown was slightly bulbous and almost smooth, unlike in other ophthalmosaurids.[3]
Postcranial skeleton

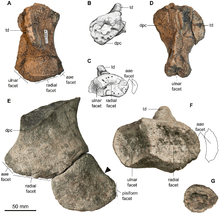
As is typical for ichthyosaurs, the vertebral centra are disc-shaped and deeply concave on both ends. Processes (bony projections for muscle and rib attachment) are greatly reduced as an adaptation for the fully aquatic lifestyle.[15] In Acamptonectes, the frontmost cervical centra (of the neck) were high and short, while the following cervical and dorsal (of the trunk) centra become progressively longer. At the rear section of the dorsal vertebral column, the centra become shorter and higher, a trend that reaches its peak at the first caudal (tail vertebrae), which is 3.12 times as high as long. The remaining caudals become longer and lower again, and the caudals that formed the fin are as long as high, a condition which is known in only one other species, Platypterygius platydactylus. The first two cervicals, the atlas and axis, are fused together to a single complex, which is wide in rear view. The front section of the dorsal (trunk) vertebral column have the diapophyses (bony sidewards projections) fused to their vertebrae; this also occurs in some other ophthalmosaurids. The centra of the dorsal vertebrae were distinct in being tightly interlocking and having extensive posterolateral lamella. In combination with the strong occiput of the skull, this interlocking resulted in a stiffening of the front section of the vertebral column. Such stiffening can be observed in other thunnosaurian ichthyosaurs, though not to the degree seen in Acamptonectes.[3]
The neural arches of the vertebrae feature narrow pre- and postzygapophyses (articular processes) that are unpaired (fused into a single element) in the whole vertebral column; this is in contrast to Platypterygius hercynicus and Sveltonectes, where these processes are paired in the front part of the column. The neural spines (upwards projections) are of variable height; in some dorsals they are markedly longer, reaching 1.25 times the height of the largest centrum. These long spines may comprise an additional element, the extraneural process, which has been described for Stenopterygius and is located above the neural spines. The top surface of the neural spines is typically pitted, indicating a cartilage covering. The ribs are distinct in being robust with a round cross-section, in contrast to the "8"-shaped cross-section seen in other thunnosaurian ichthyosaurs.[3]
Classification
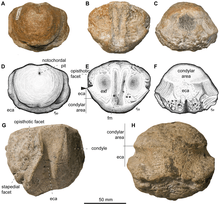
In 2012, Fischer and colleagues found Acamptonectes to be a member of the family Ophthalmosauridae based on several characteristics: the reduced extracondylar area (a band of bone surrounding the occipital condyle), the plate-like dorsal trochanter of the humerus, the presence of a facet at the front of the humerus' bottom end for a paddle bone, and the lack of notching in the paddle bones (the latter was considered to be homoplastic, or independently acquired). They also found it to be more closely related to other ophthalmosaurids than Arthropterygius based on the large processes of the basipterygoids (also bones of the braincase), the lack of a peg on the basioccipital, and the large trochanters of the femur.[3]
Relationships within the Ophthalmosauridae have historically been unstable in analyses due to the fragmentary nature of many ophthalmosaurid specimens; furthermore, many ophthalmosaurid genera are known from a single specimen. However, removal of these fragmentary genera has degraded the resolution of analyses even further.[16][17][18] The phylogenetic analysis conducted by Fischer and colleagues in 2012 recovered two novel clades (groups) within the Ophthalmosauridae, the Ophthalmosaurinae and Platypterygiinae, which had been long suspected by ichthyosaur researchers (with Maxim Arkhangelsky having named the clades as subfamilies in 2001[19]) but received no support from prior analyses.[7] Acamptonectes was placed in the former, although it represented a secondary reversal of the group's only uniting characteristic (a notch on the bottom of the basioccipital).[3]
Within the Ophthalmosaurinae, various positions have been recovered for Acamptonectes due to the same issues. In 2012, Fischer and colleagues found that it grouped closest with "Ophthalmosaurus" natans, with Ophthalmosaurus icenicus and Mollesaurus being progressively less closely related. The former clade was on account of the reduced presence of striations on the teeth, although Fischer and colleagues indicated that this characteristic was homoplastic. Thus, they did not consider it sufficient to resurrect the previously-used name Baptanodon for "O." natans.[3] In 2013, they recovered the same arrangement in a derivative analysis for the description of Malawania,[20] as did palaeontologist Nikolay Zverkov and colleagues in a 2015 analysis focusing on Grendelius (albeit with a clade consisting of Cryopterygius, Undorosaurus, and Paraophthalmosaurus being closer to Acamptonectes than Mollesaurus).[21] Arkhangelsky and Zverkov previously found all of these species (with the exception of Mollesaurus) to form an unresolved clade, or polytomy, in 2014.[22] A 2019 analysis by Zverkov and Vladimir Efimov found an otherwise identical arrangement where the positions of Mollesaurus and Acamptonectes were exchanged,[23] which was also found in another 2019 analysis from Zverkov and Natalya Prilepskaya.[24] In their description of Acuetzpalin, a 2020 analysis by Jair Barrientos-Laraa and Jesús Alvarado-Ortega found "O." natans and O. icenicus to form a clade to the exclusion of Mollesaurus and then Acamptonectes.[25]
Alternatively, a 2014 analysis by Aubrey Roberts and colleagues in the description of Janusaurus found Acamptonectes as the sister group to a clade consisting of O. icenicus and Leninia, which collectively constituted one branch of the Ophthalmosaurinae.[26] The same arrangement was recovered by a 2017 analysis conducted by Lene Delsett and colleagues, in the description of Keilhauia.[27] In 2019, another analysis from the same authors found Acamptonectes closer to Janusaurus, Keilhauia, and Palvennia than Paraophthalmosaurus, "O." natans (as Baptanodon), O. icenicus, or Gengasaurus (progressively closer to the base of the Ophthalmosaurinae in that order).[28] However, in each case, the Bremer support of the groupings (a measure of the likelihood of a phylogenetic tree's arrangement over alternatives) was low.
Other analyses still found Acamptonectes as part of polytomies. For the 2016 description of Muiscasaurus, Erin Maxwell and colleagues found O. icenicus, "O." natans, Undorosaurus, and Acamptonectes in a polytomy at the base of the Ophthalmosauridae. Contrary to most analyses, they did not recover a distinct Ophthalmosaurinae.[29] Also in 2016, Fischer and colleagues found Ophthalmosaurinae to consist of Mollesaurus as the sister group to a polytomy including O. icenicus, "O." natans, Leninia, Acamptonectes, and a group containing Cryopterygius, Janusaurus, and Palvennia.[30] In 2019, Maxwell, Dirley Cortés, Pedro Patarroyo, and Parra Ruge found a poorly-resolved Ophthalmosauridae containing Acamptonectes in a large polytomy.[31] Finally, in the 2020 description of Arthropterygius thalassonotus, Lisandro Campos and colleagues found Acamptonectes in a polytomy with O. icenicus, Leninia, and Athabascasaurus, which formed the sister group to a clade of Keilhauia and Undorosaurus; the base of the Ophthalmosaurinae was formed by a polytomy of Baptanodon, Gengasaurus, and the other species.[18]
The phylogenetic tree produced by the analysis of Barrientos-Laraa and Alvarado-Ortega in 2020 is reproduced below.[25]
| Ophthalmosauridae |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Palaeobiogeography

Ichthyosaurs were traditionally thought to have been affected by three extinction events: one at the Triassic–Jurassic boundary, one at the Jurassic–Cretaceous boundary, and a final extinction in the Cretaceous at the boundary of the Cenomanian and Turonian ages that left no survivors. Some researchers suggested that their species diversity declined after the mid-Jurassic, with the ichthyosaurs lingering on until they disappeared at the end of the Cenomanian.[7][32] This decline was thought to have been associated with a transition in the dominant ichthyosaur lineage: the large-eyed, thunniform (tuna-shaped) ophthalmosaurines, which were successful and widespread notwithstanding their hyperspecialization, would have been replaced by the more generalized platypterygiines, which had smaller eyes and longer bodies.[7][33]
Acamptonectes is a significant find in that it is an ophthalmosaurine from the Early Cretaceous, demonstrating that the ophthalmosaurines were not entirely wiped out at the Jurassic-Cretaceous boundary. Fischer and colleagues also found evidence of other ophthalmosaurines in the Early Cretaceous by reanalyzing known material; this included a basoccipital and humerus from Berriasian-aged rocks near Nettleton, Lincolnshire that they referred to Ophthalmosaurus itself. Additionally, they pointed to reports of the Late Jurassic-aged platypterygiines Brachypterygius, Aegirosaurus, Caypullisaurus, and Yasykovia (which has been synonymized with Nannopterygius[34]) from the Early Cretaceous.[3][7][35][36][37]
Lastly, by tabulating the number of genera disappearing in each age, Fischer and colleagues found that no boundary existed between ages from the Late Jurassic (Oxfordian) to Early Cretaceous (Aptian) that could be considered an extinction event for ophthalmosaurids; in particular, the Jurassic-Cretaceous boundary had a net extinction rate of 0 and even the highest survival rates. (However, by counting the number of new clades that emerged, they found that the cladogenesis rate was lower in the Cretaceous.) Thus, they concluded—contrary to traditional thinking—that the Jurassic-Cretaceous extinction event had a negligible impact on ichthyosaurs compared to other marine reptiles, with ophthalmosaurids having remained diverse until their final extinction.[3]
Palaeobiology
With their dolphin-like bodies, ichthyosaurs were better adapted to an aquatic environment than any other group of marine reptiles.[15] They were viviparous (gave birth to live young) and likely incapable of leaving the water. As homeotherms with high metabolic rates, ichthyosaurs would have been active swimmers.[38] Jurassic and Cretaceous ichthyosaurs, including Acamptonectes, had evolved thunniform (tuna-like) swimming, as opposed to the anguilliform (undulating) mode of locomotion employed by earlier species.[15] Thunniform ichthyosaurs were able to swim faster and more efficiently than other marine reptiles of similar sizes,[39] and better adapted to a pelagic lifestyle.[20] This method of swimming was aided by their more compact bodies and crescent-shaped caudal fins.[15]
Most of the skeleton of Acamptonectes appears to have been unusually rigid, which would have in effect severely limited the amount of side-to-side motion possible in the front part of the skeleton. Its snout was also shallower than in related species, and its ribs were more rounded in cross-section, which may have been a further adaptation to increase the stiffness of the animal's body, as they were likely more resistant to bending, according to the palaeontologist Darren Naish, one of the describers.[7] The tightly packed occipital bones and cervical vertebrae would have allowed little movement in the neck, indicating it must have "shot through the water like a dart", according to palaeontologist Ulrich Joger, one of the describers.[12]
Diet and feeding
As an ophthalmosaurine, Acamptonectes would likely have been an opportunistic generalist.[10] Like other ichthyosaurs, it was a predator and probably fed on fish and squid.[3][12] Their adaptations to speed suggests that these ichthyosaurs were pursuit predators.[39] In 2012, the palaeontologist Maria Zammit suggested that the slender, shallow snout and tooth morphology of Acamptonectes indicated it had a different diet and lifestyle from other known Cretaceous ichthyosaurs. The slender tooth crowns with longitudinal ridges may have been used to impale rather than grasp prey, and its diet may have consisted of fleshy prey without a hard exterior.[40] Icthyosaurs have been divided into "feeding guilds", and other examples include species that "pierced" small prey with needle-like teeth, and species that "crunched" hard-shelled prey with their more robust teeth.[11]
Ichthyosaurs had the largest eyes of any know vertebrate group,[41] and would therefore have possessed good low-light vision.[42] This would have aided with prey capture at great depths.[43] In the related genus Ophthalmosaurus, the maximum diameter of the eyeball would have been 23 centimetres (9.1 in), allowing for the detection of movement at depths of 300 m (980 ft), in the mesopelagic zone. Ophthalmosaurus could likely dive for around 20 minutes, and reach depths of 600 m (2,000 ft) or more.[44] In addition to good eyesight, the enlarged olfactory region of the brain indicates a good sense of smell.[45]
Palaeoecology
SNHM1284-R, the German specimen of Acamptonectes, comes from late Hauterivian rocks of the Lower Saxony Basin, near Cremlingen in eastern Lower Saxony.[3] The Lower Cretaceous sediments of this basin are rich in siliclastic rocks, which were deposited in the southern region of the proto-North Sea, an epicontinental sea covering much of Northwest Germany during the Lower Cretaceous. Since this region linked the warmer Tethys Sea and the colder Boreal Realm, its environment was strongly susceptible to change.[46] The late Hauterivian rocks of the region were deposited in the neritic zone, during a time of altering marine transgression and regression.[47] The surface waters were generally cool, although they sometimes increased in temperature when warmer water from the Tethys Sea entered the region.[47][6] Sedimentation rates were high, and the bottom waters were rather anoxic.[46] Organisms that inhabited this sea include dinoflagellates, ammonites, and belemnites.[47][6]
Speeton Clay
Acamptonectes is also known from Hauterivian rocks in the Speeton Clay Formation of England, within the holotype coming from the D2D beds, and the specimen NHUMK R11185 coming from the slightly older D2C beds. Material preserved in these sediments is sometimes reworked from the older Valanginian rocks below instead of actually coming from the Hauterivian. The holotype of Acamptonectes is partially articulated, as were some nearby crinoid fossils, however, indicating that Acamptonectes genuinely came from the Hauterivian.[3] The Speeton Clay Formation is composed of claystone and mudrock and is generally about 100–130 metres (330–430 ft) thick.[48][49] The δ13C levels in the Speeton Clay Formation increase during the Valanginian and continue to do so during the early Hauterivian. This is likely due to rising sea levels, as submerged land released carbon into the oceans, although the amount of δ18O increases during this time, indicating an episode of cooling.[50] δ18O levels in belemnite fossils indicate that the temperature of the Speeton clay was about 11 °C (52 °F) at the beginning of the Hauterivian, but increased to 15 °C (59 °F) during the middle part of this stage, reverting back to 11 °C (52 °F) by its end.[48] Evidence of photosynthetic organisms indicate that the Speeton Clay environment was at least partially located in the photic zone.[51]
Numerous other organisms have been recovered from the Speeton Clay Formation. Many of these were borers, including foraminiferans, fungi, chlorophyte algae, and a variety of animals, such as sponges, polychaetes, brachiopods, barnacles, bivalves, and echinoids.[51] In addition to the aforementioned crinoids,[3] invertebrates in the Speeton Clay Formation are also represented by a wide variety of ammonites and belemnites.[52][48] While fossil fish are known from the Speeton Clay, they are poorly preserved and not very abundant. These fish include both bony fish and cartilaginous fish, the latter group represented by sharks and rays of various types.[49] Marine reptiles are uncommon in this formation, and, besides Acamptonectes are represented by some fragmentary plesiosaur remains.[3]
See also
References
- Ogg, J. G.; Hinnov, L.A. (2012). "Cretaceous". In Gradstein, F.M.; Ogg, J.G.; Schmitz, M.D.; Ogg, G.M. (eds.). The Geologic Time Scale 2012. pp. 793–853. doi:10.1016/B978-0-444-59425-9.00027-5. ISBN 9780444594259.
- Lott, G.K.; Fletcher, B.N.; Wilkinson, I.P. (1986). "The stratigraphy of the Lower Cretaceous Speeton Clay Formation in a cored borehole off the coast of north-east England". Proceedings of the Yorkshire Geological Society. 46 (1): 39–56. doi:10.1144/pygs.46.1.39.
- Fischer, V.; Maisch, M. W.; Naish, D.; Kosma, R.; Liston, J.; Joger, U.; Krüger, F. J.; Pérez, J. P.; Tainsh, J.; Appleby, R. M.; Fenton, B. (2012). "New Ophthalmosaurid Ichthyosaurs from the European Lower Cretaceous Demonstrate Extensive Ichthyosaur Survival across the Jurassic–Cretaceous Boundary". PLOS ONE. 7 (1): e29234. Bibcode:2012PLoSO...729234F. doi:10.1371/journal.pone.0029234. PMC 3250416. PMID 22235274.
- Liston, J. (2012). "Missing Skull-Bones: Hidden Sea Dragon - A guest post by Jeff Liston". Mr Wood's Fossils. Retrieved 13 February 2020.
- Fischer, V.; Maisch, M.W.; Naish, D.; Kosma, R.; Liston, J.; Joger, U.; Krüger, F.J.; Pérez, J.P.; Tainsh, J.; Appleby, R.M.; Fenton, B. (2012). "Correction: New Ophthalmosaurid Ichthyosaurs from the European Lower Cretaceous Demonstrate Extensive Ichthyosaur Survival across the Jurassic–Cretaceous Boundary". PLOS ONE. 7 (1). doi:10.1371/annotation/9731f93a-c28f-4234-8fd9-c587a103b572. S2CID 215213303.
- Seibertz, E.; Krüger, F. J. (2008). "Biostratigraphie und Paläobiogeographie des Hauterivium von Cremlingen bei Braunschweig bestimmt mit Cephalopoden (Unterkreide, Ostniedersachsen)". Braunschweiger Naturkundliche Schriften. 8: 273–287.
- Naish, D. (3 January 2012). "'Rigid Swimmer' and the Cretaceous Ichthyosaur Revolution (part I)". Tetrapod Zoology. Scientific American Blogs. Archived from the original on 4 April 2012.
- Broili, F. (1909). "Neue Ichthyosaurierreste aus der Kreide Norddeustschlands und das Hypophysenloch bei Ichthyosauriern". Palaeontographica. 55: 295–302.
- McGowan, C. (1972). "The systematics of Cretaceous ichthyosaurs with particular reference to the material from North America". Rocky Mountain Geology. 11 (1): 9–29. ISSN 1555-7332.
- Fischer, Valentin; Bardet, Nathalie; Guiomar, Myette; Godefroit, Pascal (2014). "High diversity in cretaceous ichthyosaurs from Europe prior to their extinction". PLOS ONE. 9 (1): e84709. Bibcode:2014PLoSO...984709F. doi:10.1371/journal.pone.0084709. PMC 3897400. PMID 24465427.
- Marek, R. (2015). "Fossil Focus: Ichthyosaurs". Palaeontology Online. 5: 8.
- "German marine reptile find rewrites fossil record". BBC News. London: BBC. 5 January 2012.
- Lindgren, J.; Sjövall, P.; Carney, R.M.; Uvdal, P.; Gren, J.A.; Dyke, G.; Schultz, B.P.; Shawkey, M.D.; Barnes, K.R.; Polcyn, M.J. (2014). "Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles". Nature. 506 (7489): 484–488. Bibcode:2014Natur.506..484L. doi:10.1038/nature12899. PMID 24402224. S2CID 4468035.
- McGowan, C.; Motani, R. (2003). Handbook of paleoherpetology: Ichthyopterygia. Munich: Verlag Dr. Friedrich Pfeil. ISBN 3-89937-007-4.
- Sander, P.M. (2000). "Ichthyosauria: their diversity, distribution, and phylogeny". Paläontologische Zeitschrift. 74 (1–2): 1–35. doi:10.1007/BF02987949. S2CID 85352593.
- Maxwell, E.E.; Dick, D.; Padilla, S.; Parra, M.L. (2015). "A new ophthalmosaurid ichthyosaur from the Early Cretaceous of Colombia". Papers in Palaeontology. 2 (1): 59–70. doi:10.1002/spp2.1030.
- Fernandez, M.S.; Campos, L. (2015). "Ophthalmosaurids (Ichthyosauria: Thunnosauria): alpha taxonomy, clades and names". Publicación Electrónica de la Asociación Paleontológica Argentina. 15 (1).
- Campos, L.; Fernández, M.S.; Herrera, Y. (2020). "A new ichthyosaur from the Late Jurassic of north-west Patagonia (Argentina) and its significance for the evolution of the narial complex of the ophthalmosaurids". Zoological Journal of the Linnean Society. 188 (1): 180–201. doi:10.1093/zoolinnean/zlz095.
- Arkhangelsky, M.S. (2001). "The historical sequence of Jurassic and Cretaceous ichthyosaurs". Paleontological Journal. 35 (5): 521–524.
- Fischer, V.; Appleby, R.M.; Naish, D.; Liston, J.; Riding, J.B.; Brindley, S.; Godefroit, P. (2013). "A basal thunnosaurian from Iraq reveals disparate phylogenetic origins for Cretaceous ichthyosaurs". Biology Letters. 9 (4): 20130021. doi:10.1098/rsbl.2013.0021. PMID 23676653. S2CID 9981885.
- Zverkov, N.G.; Arkhangelsky, M.S.; Stenshin, I.M. (2015). "ОБЗОР ПОЗДНЕЮРСКИХ ИХТИОЗАВРОВ РОССИИ, ИМЕЮЩИХ КОНТАКТ ИНТЕРМЕДИУМА И ПЛЕЧЕВОЙ КОСТИ. ПЕРЕСМОТР СТАТУСА РОДА GRENDELIUS MCGOWAN, 1976" [On a new ichthyosaur of the genus Undorosaurus] (PDF). Proceedings of the Zoological Institute RAS. 319 (5): 558–588. ISSN 0206-0477. OCLC 7905963202.
- Arkhangelsky, M.S.; Zverkov, N.G. (2014). "О НОВОМ ПРЕДСТАВИТЕЛЕ ИХТИОЗАВРОВ РОДА UNDOROSAURUS" [On a new ichthyosaur of the genus Undorosaurus] (PDF). Proceedings of the Zoological Institute RAS. 318 (3): 187–196. ISSN 0206-0477. OCLC 7905963202.
- Zverkov, N.G.; Efimov, V.M. (2019). "Revision of Undorosaurus, a mysterious Late Jurassic ichthyosaur of the Boreal Realm". Journal of Systematic Palaeontology. 17 (14): 1183–1213. doi:10.1080/14772019.2018.1515793.
- Zverkov, N.G.; Prilepskaya, N.E. (2019). "A prevalence of Arthropterygius (Ichthyosauria: Ophthalmosauridae) in the Late Jurassic—earliest Cretaceous of the Boreal Realm". PeerJ. 7: e6799. doi:10.7717/peerj.6799. PMC 6497043. PMID 31106052.
- Barrientos-Lara, J.I.; Alvarado-Ortega, J. (2020). "Acuetzpalin carranzai gen et sp. nov. A new ophthalmosauridae (Ichthyosauria) from the Upper Jurassic of Durango, North Mexico". Journal of South American Earth Sciences. 98: 102456. Bibcode:2020JSAES..9802456B. doi:10.1016/j.jsames.2019.102456.
- Roberts, A.J.; Druckenmiller, P.S.; Sætre, G.-P.; Hurum, J.H. (2014). "A New Upper Jurassic Ophthalmosaurid Ichthyosaur from the Slottsmøya Member, Agardhfjellet Formation of Central Spitsbergen". PLOS ONE. 9 (8): e103152. Bibcode:2014PLoSO...9j3152R. doi:10.1371/journal.pone.0103152. PMC 4118863. PMID 25084533.
- Delsett, L.L.; Roberts, A.J.; Druckenmiller, P.S.; Hurum, J.H. (2017). "A New Ophthalmosaurid (Ichthyosauria) from Svalbard, Norway, and Evolution of the Ichthyopterygian Pelvic Girdle". PLOS ONE. 12 (1): e0169971. Bibcode:2017PLoSO..1269971D. doi:10.1371/journal.pone.0169971. PMID 28121995. S2CID 28124852.
- Delsett, L.L.; Robert, A.J.; Druckenmiller, P.S.; Hurum, J.H. (2019). "Osteology and phylogeny of Late Jurassic ichthyosaurs from the Slottsmoya Member Lagerstätte (Spitsbergen, Svalbard)". Acta Palaeontologica Polonica. 64 (4): 717–743. doi:10.4202/app.00571.2018.
- Maxwell, E.E.; Dick, D.; Padilla, S.; Parra, M.L. (2016). "A new ophthalmosaurid ichthyosaur from the Early Cretaceous of Colombia". Papers in Palaeontology. 2 (1): 59–70. doi:10.1002/spp2.1030.
- Fischer, V.; Bardet, N.; Benson, R.B.; Arkhangelsky, M.S.; Friedman, M. (2016). "Extinction of fish-shaped marine reptiles associated with reduced evolutionary rates and global environmental volatility". Nature Communications. 7 (1): 1–11. Bibcode:2016NatCo...710825F. doi:10.1038/ncomms10825. PMC 4786747. PMID 26953824.
- Maxwell, E.E.; Cortés, D.; Patarroyo, P.; Parra Ruge, M.L. (2019). "A new specimen of Platypterygius sachicarum (Reptilia, Ichthyosauria) from the Early Cretaceous of Colombia and its phylogenetic implications". Journal of Vertebrate Paleontology. 39 (1): e1577875. doi:10.1080/02724634.2019.1577875.
- Thorne, P.M.; Ruta, M.; Benton, M.J. (2011). "Resetting the evolution of marine reptiles at the Triassic-Jurassic boundary". Proceedings of the National Academy of Sciences. 108 (20): 8339–8344. doi:10.1073/pnas.1018959108.
- Bakker, R.T. (1993). "Plesiosaur Extinction Cycles — Events that Mark the Beginning, Middle and End of the Cretaceous". Geological Association of Canada, Special Papers. 3: 641–664.
- Zverkov, N.G.; Jacobs, M.L. "Revision of Nannopterygius (Ichthyosauria: Ophthalmosauridae): reappraisal of the 'inaccessible' holotype resolves a taxonomic tangle and reveals an obscure ophthalmosaurid lineage with a wide distribution". Zoological Journal of the Linnean Society: zlaa028. doi:10.1093/zoolinnean/zlaa028.
- Fernández, M. (2007). "Redescription and phylogenetic position of Caypullisaurus (Ichthyosauria: Ophthalmosauridae)". Journal of Paleontology. 81 (2): 368–375. doi:10.1666/0022-3360(2007)81[368:RAPPOC]2.0.CO;2.
- Fernández, M.; Aguirre-Urreta, M.B. (2005). "Revision of Platypterygius hauthali von Huene, 1927 (Ichthyosauria: Ophthalmosauridae) from the Early Cretaceous of Patagonia, Argentina". Journal of Vertebrate Paleontology. 25 (3): 583–587. doi:10.1671/0272-4634(2005)025[0583:ROPHVH]2.0.CO;2.
- Fischer, V.; Clément, A.; Guiomar, M.; Godefroit, P. (2011). "The first definite record of a Valanginian ichthyosaur and its implications on the evolution of post-Liassic Ichthyosauria". Cretaceous Research. 32 (2): 155–163. doi:10.1016/j.cretres.2010.11.005.
- Nakajima, Y.; Houssaye, A.; Endo, H. (2014). "Osteohistology of the Early Triassic ichthyopterygian reptile Utatsusaurus hataii: Implications for early ichthyosaur biology". Acta Palaeontologica Polonica. 59 (2): 343–352. doi:10.4202/app.2012.0045.
- Massare, J.A.; Buchholtz, E.A.; Kenney, J.M.; Chomat, A.-M. (2006). "Vertebral morphology of Ophthalmosaurus natans (Reptilia: Ichthyosauria) from the Jurassic Sundance Formation of Wyoming". Paludicola. 5 (4): 242–254.
- Zammit, Maria (2012). "Cretaceous Ichthyosaurs: Dwindling Diversity, or the Empire Strikes Back?". Geosciences. 2 (2): 11–24. Bibcode:2012Geosc...2...11Z. doi:10.3390/geosciences2020011. ISSN 2076-3263. OCLC 815248898. S2CID 15064470.
- Motani, R.; Rothschild, B.M.; Wahl, W. Jr. (1999). "Large eyeballs in diving ichthyosaurs". Nature. 402 (6763): 747. Bibcode:1999Natur.402..747M. doi:10.1038/45435. S2CID 5432366.
- Fischer, V.; Arkhangelsky, M.S.; Uspensky, G.N.; Stenshin, I.M.; Godefroit, P. (2014). "A new Lower Cretaceous ichthyosaur from Russia reveals skull shape conservatism within Ophthalmosaurinae" (PDF). Geological Magazine. 51 (1): 60–70. Bibcode:2014GeoM..151...60F. doi:10.1017/S0016756812000994.
- Humphries, S.; Ruxton, G.D. (2002). "Why did some ichthyosaurs have such large eyes?" (PDF). The Journal of Experimental Biology. 205 (4): 439–441. PMID 11893757.
- Motani, R. (2005). "Evolution of fish-shaped reptiles (Reptilia: Ichthyopterygia) in their physical environments and constraints". Annual Review of Earth and Planetary Sciences. 33: 395–420. Bibcode:2005AREPS..33..395M. doi:10.1146/annurev.earth.33.092203.122707. S2CID 54742104.
- Marek, R.; Moon, B.; Williams, M.; Benton, M. (2015). "The skull and endocranium of a Lower Jurassic ichthyosaur based on digital reconstructions". Palaeontology. 58 (4): 1–20. doi:10.1111/pala.12174.
- Möller, Carla; Mutterlose, Jörg (2014). "Middle Hauterivian biostratigraphy and palaeoceanography of the Lower Saxony Basin (Northwest Germany)". Zeitschrift der Deutschen Gesellschaft für Geowissenschaften. 165 (4): 501–520.
- Weinkauf, M. F. G.; Keupp, H.; Mutterlose, J. (2013). "Calcareous dinoflagellates from the Late Hauterivian (Early Cretaceous) of Frielingen, Germany". Documenta Naturae. 192 (3): 241–271.
- McArthur, J.M.; Mutterlose, J.; Price, G. D.; Rawson, P.F.; Ruffell, A.; Thirlwall, M.F. (2004). "Belemnites of Valanginian, Hauterivian and Barremian age: Sr-isotope stratigraphy, composition (87Sr/86Sr, δ13C, δ18O, Na, Sr, Mg), and palaeo-oceanography". Palaeogeography, Palaeoclimatology, Palaeoecology. 202 (3–4): 253–272. Bibcode:2004PPP...202..253M. doi:10.1016/S0031-0182(03)00638-2.
- Underwood, C.J.; Mitchell, S.F.; Veltcamp, K.J. (1999). "Shark and ray teeth from the Hauterivian (Lower Cretaceous) of north‐east England" (PDF). Palaeontology. 42 (2): 287–302. doi:10.1111/1475-4983.00074.
- Hart, M.B.; Price, G.D.; Smart, C.W. (2008). "Foraminifera and sequence stratigraphy of the lower part of the Speeton Clay Formation (Lower Cretaceous) in NE England". Annalen des Naturhistorischen Museums in Wien. Serie a für Mineralogie und Petrographie, Geologie und Paläontologie, Anthropologie und Prähistorie. 110 (A): 423–442. JSTOR 41701824.
- Taylor, Paul D.; Barnbrook, Jane A.; Sendino, Consuelo (2013). "Endolithic biota of belemnites from the Early Cretaceous Speeton Clay Formation of North Yorkshire, UK". Proceedings of the Yorkshire Geological Society. 59 (4): 227–245.
- Doyle, J.C. (1989). "The stratigraphy of a late Lower Hauterivian horizon in the Speeton Clay formation (Lower Cretaceous) of East Yorkshire". Proceedings of the Geologists' Association. 100 (2): 175–182. doi:10.1016/S0016-7878(89)80004-5.


