β-Alanine
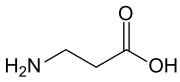
β-Alanine (or beta-alanine) is a naturally occurring beta amino acid, which is an amino acid in which the amino group is at the β-position from the carboxylate group (i.e., two atoms away, see Figure 1). The IUPAC name for β-alanine is 3-aminopropanoic acid. Unlike its counterpart α-alanine, β-alanine has no stereocenter.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Aminopropanoic acid | |
| Other names
β-Alanine 3-Aminopropionic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.215 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1][2] | |
| C3H7NO2 | |
| Molar mass | 89.093 g/mol |
| Appearance | white bipyramidal crystals |
| Odor | odorless |
| Density | 1.437 g/cm3 (19 °C) |
| Melting point | 207 °C (405 °F; 480 K) (decomposes) |
| 54.5 g/100 mL | |
| Solubility | soluble in methanol. Insoluble in diethyl ether, acetone |
| log P | -3.05 |
| Acidity (pKa) |
|
| Hazards | |
| Main hazards | Irritant |
| Safety data sheet | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1000 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis and industrial route
In terms of its biosynthesis, it is formed by the degradation of dihydrouracil and carnosine. β-Alanine ethyl ester is the ethyl ester which hydrolyses within the body to form β-alanine.[4] It is produced industrially by the reaction of ammonia with β-propiolactone.[5]
Biochemical function
β-Alanine is not found in any major proteins or enzymes. It is a component of the naturally occurring peptides carnosine and anserine and also of pantothenic acid (vitamin B5), which itself is a component of coenzyme A. Under normal conditions, β-alanine is metabolized into acetic acid.
β-Alanine is the rate-limiting precursor of carnosine, which is to say carnosine levels are limited by the amount of available β-alanine, not histidine.[6] Supplementation with β-alanine has been shown to increase the concentration of carnosine in muscles, decrease fatigue in athletes and increase total muscular work done.[7][8] Simply supplementing with carnosine is not as effective as supplementing with β-alanine alone since carnosine, when taken orally, is broken down during digestion to its components, histidine and β-alanine. Hence, by weight, only about 40% of the dose is available as β-alanine.[6]
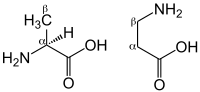
L-Histidine, with a pKa of 6.1 is a relatively weak buffer over the physiological intramuscular pH range. However, when bound to other amino acids, this increases nearer to 6.8-7.0. In particular, when bound to β-alanine, the pKa value is 6.83,[9] making this a very efficient intramuscular buffer. Furthermore, because of the position of the beta amino group, β-alanine dipeptides are not incorporated into proteins, and thus can be stored at relatively high concentrations (millimolar). Occurring at 17–25 mmol/kg (dry muscle),[10] carnosine (β-alanyl-L-histidine) is an important intramuscular buffer, constituting 10-20% of the total buffering capacity in type I and II muscle fibres.
Even though much weaker than glycine (and, thus, with a debated role as a physiological transmitter), β-alanine is an agonist next in activity to the cognate ligand glycine itself, for strychnine-sensitive inhibitory glycine receptors (GlyRs) (the agonist order: glycine ≫ β-alanine > taurine ≫ alanine, L-serine > proline).[11]
Athletic performance enhancement
There is evidence that β-alanine supplementation can increase exercise and cognitive performance, but there is concern about lack of information about safety.[12][13][14][15]
Ingestion of β-Alanine can cause paraesthesia, reported as a tingling sensation, in a dose-dependent fashion.[15]
Metabolism
Sources for β-alanine includes pyrimidine catabolism of cytosine and uracil.
β-Alanine can undergo a transamination reaction with pyruvate to form malonate-semialdehyde and L-alanine. The malonate semialdehyde can then be converted into malonate via malonate-semialdehyde dehydrogenase. Malonate is then converted into malonyl-CoA and enter fatty acid biosynthesis.[16]
Alternatively, β-alanine can be diverted into pantothenic acid and coenzyme A biosynthesis.[16]
References
- The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 196.
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-83. ISBN 0-8493-0462-8..
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 5–88. ISBN 978-1498754286.
- Wright, Margaret Robson (1969). "Arrhenius parameters for the acid hydrolysis of esters in aqueous solution. Part I. Glycine ethyl ester, β-alanine ethyl ester, acetylcholine, and methylbetaine methyl ester". Journal of the Chemical Society B: Physical Organic: 707–710. doi:10.1039/J29690000707.
- Karlheinz Miltenberger "Hydroxycarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a13_507
- "Beta-Alanine Supplementation For Exercise Performance". Retrieved 21 September 2018.
- Derave W, Ozdemir MS, Harris R, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E (August 9, 2007). "Beta-alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters". J Appl Physiol. 103 (5): 1736–43. doi:10.1152/japplphysiol.00397.2007. PMID 17690198.
- Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA (2007). "Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity". Amino Acids. 32 (2): 225–33. doi:10.1007/s00726-006-0364-4. PMID 16868650.
- Bate-Smith, EC (1938). "The buffering of muscle in rigor: protein, phosphate and carnosine". Journal of Physiology. 92 (3): 336–343. doi:10.1113/jphysiol.1938.sp003605. PMC 1395289. PMID 16994977.
- Mannion, AF; Jakeman, PM; Dunnett, M; Harris, RC; Willan, PLT (1992). "Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans". Eur. J. Appl. Physiol. 64 (1): 47–50. doi:10.1007/BF00376439. PMID 1735411.
- Encyclopedia of Life Sciences Amino Acid Neurotransmitters. Jeremy M Henley, 2001 John Wiley & Sons, Ltd. doi:10.1038/npg.els.0000010, Article Online Posting Date: April 19, 2001
- Quesnele JJ, Laframboise MA, Wong JJ, Kim P, Wells GD (2014). "The effects of beta-alanine supplementation on performance: a systematic review of the literature". Int J Sport Nutr Exerc Metab (Systematic review). 24 (1): 14–27. doi:10.1123/ijsnem.2013-0007. PMID 23918656.
- Hoffman JR, Stout JR, Harris RC, Moran DS (2015). "β-Alanine supplementation and military performance". Amino Acids. 47 (12): 2463–74. doi:10.1007/s00726-015-2051-9. PMC 4633445. PMID 26206727.
- Hobson, R. M.; Saunders, B.; Ball, G.; Harris, R. C.; Sale, C. (9 December 2016). "Effects of β-alanine supplementation on exercise performance: a meta-analysis". Amino Acids. 43 (1): 25–37. doi:10.1007/s00726-011-1200-z. ISSN 0939-4451. PMC 3374095. PMID 22270875.
- Trexler ET, Smith-Ryan AE, Stout JR, Hoffman JR, Wilborn CD, Sale C, Kreider RB, Jäger R, Earnest CP, Bannock L, Campbell B, Kalman D, Ziegenfuss TN, Antonio J (2015). "International society of sports nutrition position stand: Beta-Alanine". J Int Soc Sports Nutr (Review). 12: 30. doi:10.1186/s12970-015-0090-y. PMC 4501114. PMID 26175657.
- "KEGG PATHWAY: beta-Alanine metabolism - Reference pathway". www.genome.jp. Retrieved 2016-10-04.
