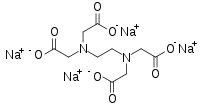
Tetrasodium EDTA
Tetrasodium EDTA is the salt resulting from the neutralization of ethylenediaminetetraacetic acid with four equivalents of sodium hydroxide (or an equivalent sodium base). It is a white solid that is highly soluble in water. Commercial samples are often hydrated, e.g. Na4EDTA.4H2O. The properties of solutions produced from the anhydrous and hydrated forms are the same, provided they are at the same pH.
 | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.000.522 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12N2Na4O8 | |
| Molar mass | 380.171 g·mol−1 |
| Appearance | White solid |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H318 |
| P264, P270, P280, P301+312, P305+351+338, P310, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is used as a source of the chelating agent EDTA4-. A 1% aqueous solution has a pH of approximately 11.3. When dissolved in neutral water, it converts partially to H2EDTA2-. Ethylenediaminetetraacetic acid is produced commercially via the intermediacy of tetrasodium EDTA.[1]
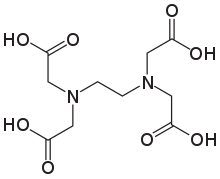
Ethylenediaminetetraacetic acid is the acidic form of tetrasodium EDTA.
References
- Hart, J. Roger. "Ethylenediaminetetraacetic Acid and Related Chelating Agents". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_095.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.