Retinol dehydrogenase
In enzymology, a retinol dehydrogenase (RDH) (EC 1.1.1.105) is an enzyme that catalyzes the chemical reaction
- retinol + NAD+ retinal + NADH + H+
| Retinol dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.1.1.105 | ||||||||
| CAS number | 9033-53-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Sometimes, in addition to or along with NAD+, NADP+ can act as a preferred cofactor in the reaction as well. The substrate of the enzyme can be all-trans- or -cis- retinol. There are at least over 20 different isolated enzymes with RDH activity to date.[1] Thus, the two substrates of this enzyme are retinol and NAD+, whereas its 3 products are retinal, NADH (or NADPH in the case where NADP+ is a cofactor), and H+.[1]
This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is retinol:NAD+ oxidoreductase. Other names in common use include retinol (vitamin A1) dehydrogenase, MDR, microsomal retinol dehydrogenase, all-trans retinol dehydrogenase, retinal reductase, and retinene reductase. This enzyme participates in retinol metabolism.[2] Occasionally, the literature refers to retinol dehydrogenase as an enzyme that oxidizes retinol in general, such as class IV alcohol dehydrogenase (ADH4), which reportedly is the most efficient retinol oxidation in the human alcohol dehydrogenase (ADH) family.[3][4]
Structure
As one of the most important RDH, 11-cis-retinol dehydrogenase catalyzes the 11-cis retinaldehyde (the most common visual pigments in higher animals) formation. The enzyme is mainly expressed in the retinal pigment epithelium (RPE) and is part of short-chain dehydrogenase (SDR) / reductase superfamily. The integral membrane enzyme is anchored to the membranes by its two hydrophobic chains. The catalytic domain of 11-cis-retinol dehydrogenase is restricted to the lumenal compartment, suggesting its origin from compartmentalized process. 11-cis-retinol dehydrogenase is also mainly associated to the smooth endoplasmic reticulum of RPE cells.[7] The 32-kDa integral membrane protein protein (p32) was found to act as the stereospecific 11-cis-retinol dehydrogenase in the presence of NAD+ cofactor, and p32 catalyzes the biosynthesis of 11-cis retinal commonly found visual chromophore.[8]
One of the widely studied genes of retinol dehydrogenase RDH12, which encodes retinol dehydrogenase is part of the superfamily of short-chained alcohol dehydrogenases and reductases. RDH12 is mainly expressed in neuroretina and is composed of 7 exons encoding a 360-amino acid peptide.[9]
Zinc molecules serve as the ligand cofactor with the cofactor NAD. The retinol will interact with the enzyme at the area between those two cofactors.[6]
However, not all retinol dehydrogenases in visual cycle are identified, and this remains challenging to scientists due to the overlapping expressions and activity redundancy among two large RDH and RDH-like producing classes: microsomal short-chain dehydrogenase/reductase and cytosolic medium-chain alcohol dehydrogenases.[10]
In Bovine, retinol dehydrogenase is found as a part of retinal rod outer segments and shows difficulty when separating from membrane. Its Stokes radius is 8.5 nm in Lubrol 12A9 mixed micelle.[11]
Function
Retinoid dehydrogenases/reductases (oxidoreductases), including retinol dehydrogenase, catalyze the key oxidation-reduction reactions in the visual cycle, converting vitamin A to 11-cis retinal, which is the chromophore of the rod and cone photoreceptors. It is believed that RDHs at rod and cone are different, but related and can catalyze the same reaction. RDH12 is the primary enzyme that reduces all-trans retinal released from bleached photopigments during recovery phase in the visual cycle. The RDH12 enzyme can use either cis or trans retinoid isomers as substrates and can also function as both dehydrogenase (i.e. retinol to retinal) and reductase (i.e. retinal to retinol).[12][13]
The conversion of retinol to retinal is the rate-limiting step in the retinoic acid biosynthesis. In vertebrates, the retinoic acid is the ligand that controls nuclear receptor signaling pathway, which is responsible for growth and development as well as epithelial maintenance, therefore can be used for cancer and acne treatment. In human, ADH4 can exhibit at least 10 folds higher Vmax/Km than other ADH.[4]
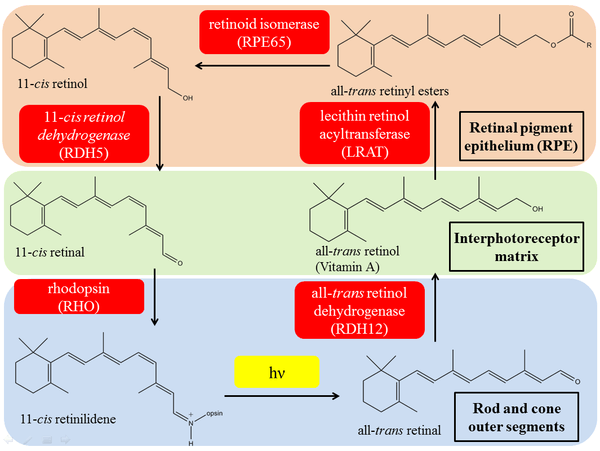
Some retinol dehydrogenases are in extra-ocular tissues, such as human retinol dehydrogenase-4 (RoDH-4), which converts retinol and 1-cis-retinol to different aldehydes in liver and skin. It was also found that 13-cis-retinoic acid (isotretinoin), 3,4-didehydroretinoic acid, and 3,4-didehydroretinol can act as competitive inhibitor of the 3α-hydroxysteroid dehydrogenase oxidative activity of the enzyme. This can potentially explain how isotretinoin, the active ingredient is Roaccutane (Accutane), can suppress sebaceous glands and be used for severe acne treatment.[15]
Disease relevance
The missense mutation in gene rdh5, which codes for microsomal 11-cis-retinol dehydrogenase (RDH5), causes fundus albipunctatus, whose symptoms include retinal white-spot accumulation, stationary night blindness caused by delay in cone and rod photopigment regeneration, and elderly cone dystrophy.[1][16]
At least 20 mutations in rdh12 gene, which encodes retinol dehydrogenase, can be associated to diseases, including severe and early-onset autosomal recessive retinal dystrophy (arRD), or Leber congenital amaurosis. Patients suffer from cone and rod malfunction since childhood and develop legal blindness when reaching adulthood. This suggests that RDH12 might play a central role in the visual cycle and can be a promising therapeutic target.[9][12] A possible mechanism of the accelerated degradation among RDH12 mutants is the polyubiquitination by cytosolic ubiquitin ligases and subsequent degradation by proteosome. Its conformation aberration provokes the aforementioned accelerated degradation.[17]
References
- Lidén M, Eriksson U (May 2006). "Understanding retinol metabolism: structure and function of retinol dehydrogenases". J. Biol. Chem. 281 (19): 13001–4. doi:10.1074/jbc.R500027200. PMID 16428379.
- Koen AL, Shaw CR (1966). "Retinol and alcohol dehydrogenases in retina and liver". Biochim. Biophys. Acta. 128 (1): 48–54. doi:10.1016/0926-6593(66)90140-8. PMID 5972368.
- Satre MA, Zgombić-Knight M, Duester G (June 1994). "The complete structure of human class IV alcohol dehydrogenase (retinol dehydrogenase) determined from the ADH7 gene". J. Biol. Chem. 269 (22): 15606–12. PMID 8195208.
- Chou CF, Lai CL, Chang YC, Duester G, Yin SJ (July 2002). "Kinetic mechanism of human class IV alcohol dehydrogenase functioning as retinol dehydrogenase". J. Biol. Chem. 277 (28): 25209–16. doi:10.1074/jbc.M201947200. PMID 11997393.
- Xie PT, Hurley TD (December 1999). "Methionine-141 directly influences the binding of 4-methylpyrazole in human sigma sigma alcohol dehydrogenase". Protein Sci. 8 (12): 2639–44. doi:10.1110/ps.8.12.2639. PMC 2144219. PMID 10631979.
- "RCSB PDB-101".
- Simon A, Romert A, Gustafson AL, McCaffery JM, Eriksson U (February 1999). "Intracellular localization and membrane topology of 11-cis retinol dehydrogenase in the retinal pigment epithelium suggest a compartmentalized synthesis of 11-cis retinaldehyde". J. Cell Sci. 112 (4): 549–58. PMID 9914166.
- Simon A, Hellman U, Wernstedt C, Eriksson U (January 1995). "The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases". J. Biol. Chem. 270 (3): 1107–12. doi:10.1074/jbc.270.3.1107. PMID 7836368.
- Janecke AR, Thompson DA, Utermann G, et al. (August 2004). "Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy". Nat. Genet. 36 (8): 850–4. doi:10.1038/ng1394. PMID 15258582.
- Farjo KM, Moiseyev G, Takahashi Y, Crouch RK, Ma JX (November 2009). "The 11-cis-retinol dehydrogenase activity of RDH10 and its interaction with visual cycle proteins". Invest. Ophthalmol. Vis. Sci. 50 (11): 5089–97. doi:10.1167/iovs.09-3797. PMID 19458327.
- Nicotra C, Livrea MA (October 1982). "Retinol dehydrogenase from bovine retinal rod outer segments. Kinetic mechanism of the solubilized enzyme". J. Biol. Chem. 257 (19): 11836–41. PMID 6749847.
- Thompson DA, Janecke AR, Lange J, et al. (December 2005). "Retinal degeneration associated with RDH12 mutations results from decreased 11-cis retinal synthesis due to disruption of the visual cycle". Hum. Mol. Genet. 14 (24): 3865–75. doi:10.1093/hmg/ddi411. PMID 16269441.
- Maeda A, Maeda T, Imanishi Y, et al. (May 2005). "Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo". J. Biol. Chem. 280 (19): 18822–32. doi:10.1074/jbc.M501757200. PMC 1283069. PMID 15755727.
- "upload.wikimedia.org".
- Karlsson T, Vahlquist A, Kedishvili N, Törmä H (March 2003). "13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands?". Biochem. Biophys. Res. Commun. 303 (1): 273–8. doi:10.1016/S0006-291X(03)00332-2. PMID 12646198.
- Yamamoto H, Simon A, Eriksson U, Harris E, Berson EL, Dryja TP (June 1999). "Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus". Nat. Genet. 22 (2): 188–91. doi:10.1038/9707. PMID 10369264.
- Lee SA, Belyaeva OV, Kedishvili NY (February 2010). "Disease-associated variants of microsomal retinol dehydrogenase 12 (RDH12) are degraded at mutant-specific rates". FEBS Lett. 584 (3): 507–10. doi:10.1016/j.febslet.2009.12.009. PMC 2812597. PMID 20006610.