Phosphite ester
In chemistry a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of an unobserved tautomer phosphorous acid, H3PO3, with the simplest example being trimethylphosphite, P(OCH3)3. Some phosphites can be considered esters of the dominant tautomer of phosphorous acid (HP(O)(OH)2). The simplest representative is dimethylphosphite with the formula HP(O)(OCH3)2. Both classes of phosphites are usually colorless liquids.
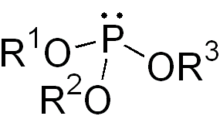
Synthesis
- From PCl3
Phosphite esters are typically prepared by treating phosphorus trichloride with an alcohol. Depending on the synthetic details, this alcoholysis can give the diorganophosphites:[1]
- PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl
Alternatively, when the alcoholysis is conducted in the presence of proton acceptors, one obtains the C3-symmetric trialkoxy derivatives:[2]
- PCl3 + 3 C2H5OH + 3 R3N → (C2H5O)3P + 3 R3NHCl
Numerous derivatives have been prepared for both types of phosphites.
- By transesterification
Phosphite esters can also be prepared by transesterification, as they undergo alcohol exchange upon heating with other alcohols.[3] This process is reversible and can be used to produce mixed alkyl phosphites. Alternatively, if the phosphite of a volatile alcohol is used, such as trimethyl phosphite, then the by product (methanol) can be removed by distillation, allowing the reaction to be driven to completion.
Reactions and applications of tris(organo)phosphites
Reactions
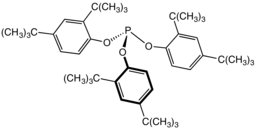 Tris(2,4-di-tert-butylphenyl)phosphite, a widely used stabilizer in polymers
Tris(2,4-di-tert-butylphenyl)phosphite, a widely used stabilizer in polymers
Phosphites are oxidized to phosphate esters:
- P(OR)3 + [O] → OP(OR)3
This reaction underpins the commercial use of some phosphite esters as stabilizers in polymers.[4]
Alkyl phosphite esters are used in the Perkow reaction for the formation of vinyl phosphonates, and in the Michaelis–Arbuzov reaction to form phosphonates. Aryl phosphite esters may not undergo these reactions and hence are commonly used as stabilizers in halogen-bearing polymers such as PVC.
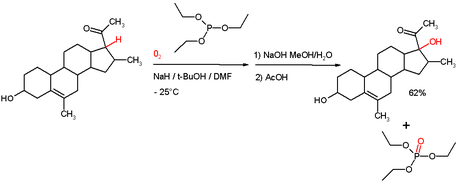
Phosphite esters may be used as reducing agents in more specialised cases. For example, triethylphosphite is known to reduce certain hydroperoxides to alcohols formed by autoxidation[5] (scheme). In this process the phosphite is converted to a phosphate ester. This reaction type is also utilized in the Wender Taxol total synthesis.
Homogeneous catalysis
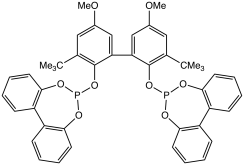
Phosphite esters are Lewis bases and hence can form coordination complexes with various metal ions. Representative phosphite ligands include trimethylphosphite ((MeO)3P), triethylphosphite ((EtO)3P), trimethylolpropane phosphite, and triphenylphosphite ((PhO)3P). Phosphites exhibit a smaller ligand cone angles than the structurally related phosphine ligand family. Phosphite ligands are components of industrial catalysts for hydroformylation and hydrocyanation.[6]
Chemistry of HP(O)(OR)2
2POH.png) Structure of a diorganophosphite.
Structure of a diorganophosphite.
Diorganophosphites are derivatives of phosphorus(V) and can be viewed as the di-esters of phosphorous acid. They exhibit tautomerism, however the equilibrium overwhelmingly favours the right-hand (phosphonate-like) form:[9]
- (RO)2POH ⇌ (RO)2P(O)H
The P-H bond is the site of high reactivity in these compounds (for example in the Atherton–Todd reaction), whereas in tri-organophosphites the lone pair on phosphorus is the site of high reactivity. Diorganophosphites do however undergo transesterification.
See also
- Phosphinite P(OR)R2
- Phosphonite P(OR)2R
- Ortho ester CH(OR)3
- Borate ester B(OR)3
References
- Malowan, John E. (1953). "Diethyl phosphite". Inorganic Syntheses. 4: 58–60. doi:10.1002/9780470132357.ch19.
- A. H. Ford-Moore; B. J. Perry (1963). "Triethyl Phosphite". Organic Syntheses.; Collective Volume, 4, p. 955
- Hoffmann, Friedrich W.; Ess, Richard J.; Usingef, Robert P. (November 1956). "The Transesterification of Trialkyl Phosphites with Aliphatic Alcohols". Journal of the American Chemical Society. 78 (22): 5817–5821. doi:10.1021/ja01603a026.
- Rainer Wolf; Bansi Lal Kaul (2000). "Plastics, Additives". Ullmann's Encyclopedia Of Industrial Chemistry. doi:10.1002/14356007.a20_459.
- J. N. Gardner; F. E. Carlon & O. Gnoj (1968). "One-step procedure for the preparation of tertiary α-ketols from the corresponding ketones". J. Org. Chem. 33 (8): 3294–3297. doi:10.1021/jo01272a055.
- Aitor Gual; Cyril Godard; Verónica de la Fuente; Sergio Castillón (2012). "Design and Synthesis of Phosphite Ligands for Homogeneous Catalysis". In Paul C. J. Kamer; Piet W. N. M. van Leeuwen (eds.). Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis. John Wiley & Sons. doi:10.1002/9781118299715.ch3.
- Cuny, Gregory D.; Buchwald, Stephen L. (1993). "Practical, High-Yield, Regioselective, Rhodium-Catalyzed Hydroformylation of Functionalized α-olefins". Journal of the American Chemical Society. 115 (5): 2066–2068. doi:10.1021/ja00058a079.
- Van Rooy, Annemiek; Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M.; Goubitz, Kees; Fraanje, Jan; Veldman, Nora; Spek, Anthony L. (1996). "Bulky Diphosphite-Modified Rhodium Catalysts: Hydroformylation and Characterization". Organometallics. 15 (2): 835–847. doi:10.1021/OM950549K.
- Doak, G. O.; Freedman, Leon D. (1 February 1961). "The Structure and Properties of the Dialkyl Phosphonates". Chemical Reviews. 61 (1): 31–44. doi:10.1021/cr60209a002.