Hydrocyanation
Hydrocyanation is, most fundamentally, the process whereby H+ and –CN ions are added to a molecular substrate. Usually the substrate is an alkene and the product is a nitrile. When –CN is a ligand in a transition metal complex, its basicity makes it difficult to dislodge, so, in this respect, hydrocyanation is remarkable. Since cyanide is both a good σ–donor and π–acceptor its presence accelerates the rate of substitution of ligands trans from itself, the trans effect.1 A key step in hydrocyanation is the oxidative addition of hydrogen cyanide to low–valent metal complexes.[1] In hydrocyanation of unsaturated carbonyls addition over the alkene competes with addition over the carbonyl group.
Stoichiometry and mechanism
Industrially, hydrocyanation is commonly performed on alkenes catalyzed by nickel complexes of phosphite (P(OR)3) ligands. A general reaction is shown:[2]
- RCH=CH2 + HCN → RCH2-CH2-CN
The reaction proceeds via the oxidative addition of HCN to Ni(0) to give a hydridonickel(II) cyanide complex, abbreviated Ni(H)(CN)L2. Subsequent binding of the alkene gives the intermediate Ni(H)(CN)L(alkene), which then undergoes migratory insertion to give an alkylnickel(II) cyanide Ni(R)(CN)L2. The cycle is completed by the reductive elimination of the nitrile.
Lewis acids, such as B(C6H5)3, can increase hydrocyanation rates and allow for lower operating temperatures. Triphenylboron may derive this ability induce reductive elimination of the nitrile product.[2] Rates can also be amplified with electron–withdrawing groups (NO2, CF3, CN, C(=O)OR, C(=O)R) on the phosphite ligands, because they stabilize Ni(0).[2]
Catalyst deactivation
Catalyst deactivation involves formation of dicyanonickel(II) species, which are unreactive toward alkenes. The dicyanide arises via two pathways (L = phosphite):[2]
- Ni(H)(CN)L2 + HCN → Ni(CN)2L2 + H2
- Ni(R)(CN)L2 + HCN → Ni(CN)2L2 + RH
Asymmetric hydrocyanation
Most alkenes are prochiral, meaning in this context that their hydrocyanation generates chiral nitriles. Conventional hydrocyanation catalysts, e.g. Ni(P(OR)3)4, catalyse the formation of racemic mixtures, consisting of equal amounts of the two enantiomeric nitriles. When however the supporting ligands are chiral, the hydrocyanation can be highly enantioselective. For asymmetric hydrocyanation, popular chiral ligands are chelating aryl diphosphite complexes.[2][3][4]
Transhydrocyanation
In transhydrocyanation, an equivalent of HCN is transferred from a cyanohydrin, e.g. acetone cyanohydrin, to another HCN acceptor. The transfer is an equilibrium process, initiated by base. The reaction can be driven by trapping reactions or by the use of a superior HCN acceptor, such as an aldehyde.[5]
Applications
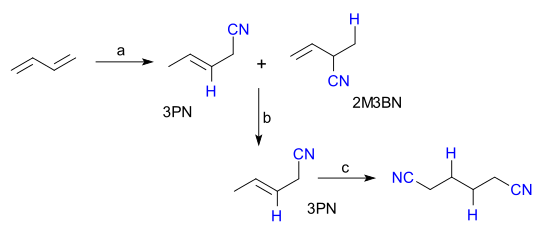
Hydrocyanation is important due to the versatility of alkyl nitriles (RCN), which are important intermediates for the syntheses of amides, amines, carboxylic acids and esters. The most important industrial application is the nickel-catalyzed synthesis of adiponitrile (NC–(CH2)4–CN) synthesis from buta-1,3-diene (CH2=CH–CH=CH2). Adiponitrile is a precursor to hexamethylenediamine (H2N–(CH2)6–NH2), which is used for the production of certain kinds of Nylon. The DuPont ADN process to give adiponitrile is shown below:
This process consists of three steps: hydrocyanation of butadiene to a mixture of 2-methyl-butene-3-nitrile (2M3BM) and pentene-3-nitrile (3PN), an isomerization step from 2M3BM (not desired) to 3PN and a second hydrocyanation (aided by a Lewis acid cocatalyst such as aluminium trichloride or triphenylboron) to adiponitrile.[6]
Asymmetric hydrocyanation
Naproxen, an anti-inflammatory drug, is prepared via an asymmetric hydrocyanation of a vinylnaphthalene utilizing a phosphinite (OPR2) ligand, L . The enantioselectivity of this reaction is important because only the S enantiomer is medicinally desirable, whereas the R enantiomer produces harmful health effects. This reaction can produce the S enantiomer with >90% stereoselectivity. Upon recrystallization of the crude product, the optically pure nitrile can be obtained.
History
Hydrocyanation was first reported by Arthur and Pratt in 1954, when they homogeneously catalyzed the hydrocyanation of linear alkenes.[7] The industrial process for catalytic hydrocyanation of butadiene to adiponitrile was invented by William C. Drinkard.
References
- Cotton, F. A.; Wilkinson, G.; Murillo, C. A.; Bochmann, M. Advanced Inorganic Chemistry; John Wiley & Sons: New York, 1999; pp. 244-6, 440, 1247-9.
- Piet W.N.M. van Leeuwen "Homogeneous Catalysis: Understanding the Art", 2004, Wiley-VCH, Weinheim. ISBN 1-4020-2000-7
- RajanBabu, T. V.; Casalnuovo, A. L. (1994). "Electronic effects in asymmetric catalysis: Enantioselective carbon-carbon bond forming processes". Pure Appl. Chem. 66 (7): 1535–42. doi:10.1351/pac199466071535.
- Goertz, Wolfgang; Kamer, Paul C. J.; van Leeuwen, Piet W. N. M.; Vogt, Dieter (1997). "Application of chelating diphosphine ligands in the nickel-catalysed hydrocyanation of alk-l-enes & ω-unsaturated fatty acid esters". Chem. Commun. (16): 1521–1522. doi:10.1039/a702811c.
- Serkos A. Haroutounian (2001). "Acetone Cyanohydrin". Encyclopedia of Reagents for Organic Synthesis. eEROS. doi:10.1002/047084289X.ra014. ISBN 978-0471936237.
- Bini, L.; Muller, C.; Wilting, J.; von Chrzanowski, L.; Spek, A. L.; Vogt, D. (2007). "Highly Selective Hydrocyanation of Butadiene toward 3-Pentenenitrile". J. Am. Chem. Soc. 129 (42): 12622–3. doi:10.1021/ja074922e. hdl:1874/26892. PMID 17902667.
- Arthur, P.; England, D. C.; Pratt, B. C.; Whitman, G. M. (1954). "Addition of Hydrogen Cyanide to Unsaturated Compounds". Journal of the American Chemical Society. 76 (21): 5364–5367. doi:10.1021/ja01650a034. ISSN 0002-7863.