Atherton–Todd reaction
The Atherton-Todd reaction is a name reaction in organic chemistry, which goes back to the British chemists F. R. Atherton, H. T. Openshaw and A. R. Todd. These described the reaction for the first time in 1945 as a method of converting dialkyl phosphites into dialkyl chlorophosphates.[1] The diacyl chlorophosphates formed are often too reactive to be isolated, though. For this reason, the synthesis of phosphates or phosphoramidates can follow the Atherton-Todd reaction in the presence of alcohols or amines. The following equation gives an overview over the Atherton-Todd reaction using the reactant dimethyl phosphite as an example:
.svg.png)
| Atherton–Todd reaction | |
|---|---|
| Named after | Frank R. Atherton Alexander R. Todd |
| Reaction type | Substitution reaction |
The reaction takes place after the addition of tetrachloromethane and a base. This base is usually a primary, secondary or tertiary amine. Instead of methyl groups other alkyl or benzyl groups may be present.
Reaction mechanism
A possible reaction mechanism for the Atherton-Todd reaction is presented here for the example of dimethylphosphite, just like in the overview reaction:[2]
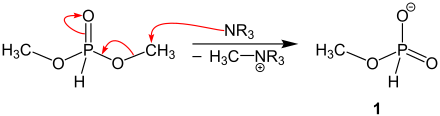
First, a tertiary amine is used to cleave a methyl group of dimethyl phosphite. The intermediate 1 results from this reaction step.
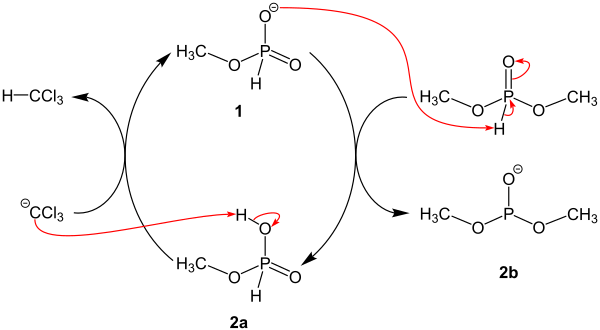
Subsequently, the intermediate 1 deprotonates the starting compound dimethylphosphite, so that intermediates 2a and intermediates 2b are formed. The intermediate 1 is then regenerated from the intermediate 2a.
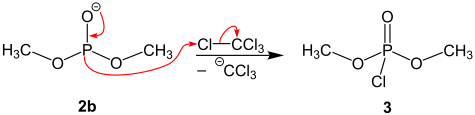
Finally, intermediate 2b is chlorinated by tetrachloromethane and dimethyl chlorophosphate 3 is formed.
Possible subsequent reactions
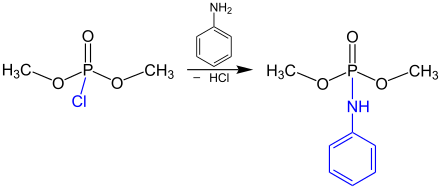
After the synthesis of the dimethyl chlorophosphate, a further reaction (for example with a primary amine like aniline) is possible by the following reaction equation:[3]
Atom economy
In this reaction, in addition to the starting compound dialkyl phosphite, tetrachloromethane and a base (an amine) are used in stoichiometric amounts. Only chloroform, which occurs after two reaction steps from tetrachloromethane, is relevant as a waste product for the assessment of the atomic economy. It should furthermore be kept in mind that the product of the reaction has a greater molar mass than the starting compound. The atom economy of this reaction can therefore be classified as relatively good.
See also
The Atherton-Todd reaction is related to the Appel reaction. In it, tetrachloromethane is used for chlorination as well.[2]
References
- F. R. Atherton, H. T. Openshaw, A. R. Todd (1945), "174. Studies on phosphorylation. Part II. The reaction of dialkyl phosphites with polyhalogen compounds in presence of bases. A new method for the phosphorylation of amines", Journal of the Chemical Society (Resumed) (in German), pp. 660–663, doi:10.1039/jr9450000660CS1 maint: multiple names: authors list (link)
- Zerong Wang (2009), Comprehensive organic name reactions and reagents Volume 1 (in German), Hoboken (N.J.): John Wiley, pp. 114–118, ISBN 978-0-470-28662-3
- Stéphanie S. Le Corre, Mathieu Berchel, Hélène Couthon-Gourvès, Jean-Pierre Haelters, Paul-Alain Jaffrès (2014), "Atherton–Todd reaction: mechanism, scope and applications", Beilstein Journal of Organic Chemistry (in German), 10 (1), pp. 1166–1196, doi:10.3762/bjoc.10.117, PMC 4077366CS1 maint: multiple names: authors list (link)