Nucleoside phosphoramidite
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers.[1] To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected. As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids. To be incorporated in the middle of an oligonucleotide chain using phosphoramidite strategy, the nucleoside analog must possess two hydroxy groups or, less often, a hydroxy group and another nucleophilic group (amino or mercapto). Examples include, but are not limited to, alternative nucleotides, LNA, morpholino, nucleosides modified at the 2'-position (OMe, protected NH2, F), nucleosides containing non-canonical bases (hypoxanthine and xanthine contained in natural nucleosides inosine and xanthosine, respectively, tricyclic bases such as G-clamp,[2] etc.) or bases derivatized with a fluorescent group or a linker arm.
Preparation
There are three main methods for the preparation of nucleoside phosphoramidites.
- The common method involves treatment of a protected nucleoside bearing a single free hydroxy group with phosphorodiamidite under the catalytic action of a weak acid.[3][4] Although some bisamidites were reported as thermally unstable compounds,[5] 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite, the amidite used to prepare commercial nucleoside phosphoramidites is relatively stable. It can be synthesized using a two-step, one-pot procedure and purified by vacuum distillation.[6] An excellent review outlines the use of the latter reagent in preparation of nucleosidic and non-nucleosidic phosphoramidites in great detail.[7]
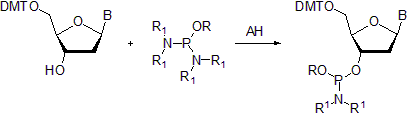 DMT = 4,4'-dimethoxytrityl; B = optionally protected nucleic base; R = phosphate protecting group
DMT = 4,4'-dimethoxytrityl; B = optionally protected nucleic base; R = phosphate protecting group
- In the second method, the protected nucleoside is treated with the phosphorochloridite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base).[8]
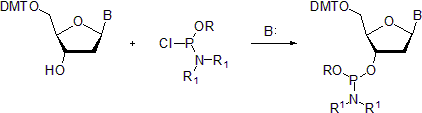
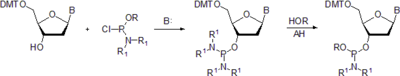
- In the third method,[9] the protected nucleoside is first treated with chloro N,N,N',N'-tetraisopropyl phosphorodiamidite in the presence of an organic base, most commonly N-ethyl-N,N-diisopropylamine (Hunig's base) to form a protected nucleoside diamidite. The latter is treated with an alcohol respective to the desired phosphite protecting group, for instance, 2-cyanoethanol, in the presence of a weak acid.
Nucleoside phosphoramidites are purified by column chromatography on silica gel. To warrant the stability of the phosphoramidite moiety, it is advisable to equilibrate the column with an eluent containing 3 to 5% of triethylamine and maintain this concentration in the eluent throughout the entire course of the separation. The purity of a phosphoramidite may be assessed by 31P NMR spectroscopy. As the P(III) atom in a nucleoside phosphoramidite is chiral, it displays two peaks at about 149 ppm corresponding to the two diastereomers of the compound. The potentially present phosphite triester impurity displays peak at 138–140 ppm. H-phosphonate impurities display peaks at 8 and 10 ppm.
Chemical properties of phosphoramidite moiety
Nucleoside phosphoramidites are relatively stable compounds with a prolonged shelf-life when stored as powders under anhydrous conditions in the absence of air at temperatures below 4 °C. The amidites withstand mild basic conditions. In contrast, in the presence of even mild acids, phosphoramidites perish almost instantaneously. The phosphoramidites are relatively stable to hydrolysis under neutral conditions. For instance, half-life of 2-cyanoethyl 5'-O-(4,4'-dimethoxytrityl)thymidine-3'-O-(N,N-diisopropylamino)phosphite in 95% aqueous acetonitrile at 25 °C is 200 h.[10]
- The most important feature of phosphoramidites is their ability to undergo the phosphoramidite coupling reaction that is, to react with nucleophilic groups in the presence of an acidic azole catalyst, 1H-tetrazole, 2-ethylthiotetrazole,[11] 2-benzylthiotetrazole,[12][13] 4,5-dicyanoimidazole,[14] or a number of similar compounds. The reaction proceeds extremely rapidly. This very feature makes nucleoside phosphoramidites useful intermediates in oligonucleotide synthesis. Stereochemically, the phosphoramidite coupling leads to the epimerisation (forming of diastereomers) at the P(III) chiral center.
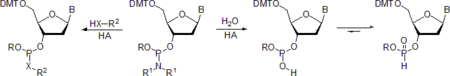 X = O, S, NH.
X = O, S, NH.
When water is served as a nucleophile, the product is an H-phosphonate diester as shown in Scheme above. Due to the presence of residual water in solvents and reagents, the formation of the latter compound is the most common complication in the preparative use of phosphoramidites, particularly in oligonucleotide synthesis.
- Phosphoramidites are readily oxidized with weak oxidating reagents, for instance, with aqueous iodine in the presence of weak bases or with hydrogen peroxide[15] to form the respective phosphoramidates.
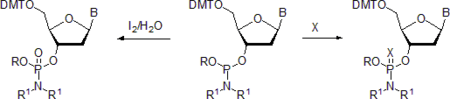 X = S, Se.
X = S, Se.
Similarly, phosphoramidites react with other chalcogens. When brought in contact with a solution of sulfur[15][16] or a number of compounds collectively referred to as sulfurizing agents,[17][18] phosphoramidites quantitatively form phosphorothioamidates. The reaction with selenium[15][16] or selenium derivatives[19] produces phosphoroselenoamidates. In all reactions of this type, the configuration at the phosphorus atom is retained.
- Nucleoside phosphoramidites undergo Michaelis-Arbuzov reaction to form the respective phosphonamidates. One example describes the preparation of phosphonamidates in the presence of acrylonitrile.[20] Reportedly, at room temperature the reaction is stereoselective with the retention of configuration at the phosphorus center. In contrast, when carried out at 55 °C, the reaction leads to racemized products.
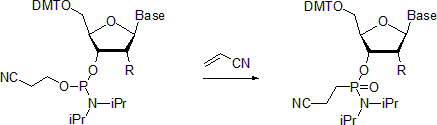
- Similarly to phosphines and tertiary phosphites, phosphoramidites readily undergo Staudinger reaction.
(RO)2P-N(R1)2 + R2-N3 + H2O ---- (RO)2P(=O)-N(R1)2 + R2-NH2 + N2;
Protecting strategy
The naturally occurring nucleotides (nucleoside-3'- or 5'-phosphates) and their phosphodiester analogs are insufficiently reactive to afford an expeditious synthetic preparation of oligonucleotides in high yields. The selectivity and the rate of the formation of internucleosidic linkages are dramatically improved by using 3'-O-(N,N-diisopropyl phosphoramidite) derivatives of nucleosides (nucleoside phosphoramidites) that serve as building blocks in phosphite triester methodology. To prevent undesired side reactions, all other functional groups present in nucleosides must be rendered unreactive (protected) by attaching protecting groups. Upon the completion of the oligonucleotide chain assembly, all the protecting groups are removed to yield the desired oligonucleotides. Below, the protecting groups currently used in commercially available[21][22][23][24][25] and most common nucleoside phosphoramidite building blocks are briefly reviewed:
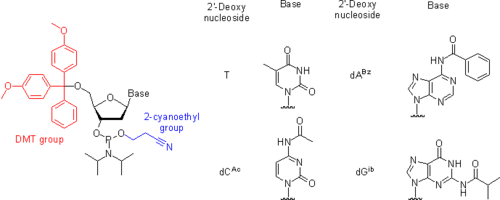
- The 5'-hydroxyl group is protected by an acid-labile DMT (4,4'-dimethoxytrityl) group.
- Thymine and uracil, nucleic bases of thymidine and uridine, respectively, do not have exocyclic amino groups and hence do not require any protection. In contrast, nucleic bases adenine, cytosine, and guanine bear the exocyclic amino groups, which are reactive with the activated phosphoramidites under the conditions of the coupling reaction. Although, at the expense of additional steps in the synthetic cycle, the oligonucleotide chain assembly may be carried out using phosphoramidites with unprotected amino groups,[26] most often these are kept permanently protected over the entire length of the oligonucleotide chain assembly. The protection of the exocyclic amino groups must be orthogonal to that of the 5'-hydroxy group because the latter is removed at the end of each synthetic cycle. The simplest to implement and hence the most widely accepted is the strategy where the exocyclic amino groups bear a base-labile protection. Most often, two protection schemes are used.
- In the first, the standard and more robust scheme (Figure), Bz (benzoyl) protection is used for A, dA, C, dC, G, and dG are protected with isobutyryl group. More recently, Ac (acetyl) group is often used to protect C and dC as shown in Figure.[27]
- In the second, mild protection scheme, A and dA are protected with isobutyryl[28] or phenoxyacetyl groups (PAC).[29] C and dC bear acetyl protection,[27] and G and dG are protected with 4-isopropylphenoxyacetyl (i-Pr-PAC)[30] or dimethylformamidino (dmf)[31] groups. Mild protecting groups are removed more readily than the standard protecting groups. However, the phosphoramidites bearing these groups are less stable when stored in solution.
- The phosphite group is protected by a base-labile 2-cyanoethyl group.[32] Once a phosphoramidite has been coupled to the solid support-bound oligonucleotide and the phosphite moieties have been converted to the P(V) species, the presence of the phosphate protection is not mandatory for the successful conducting of further coupling reactions.[33]
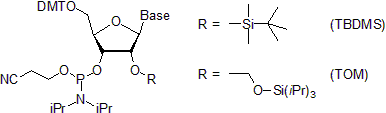
- In RNA synthesis, the 2'-hydroxy group is protected with TBDMS (t-butyldimethylsilyl) group.[34][35][36][37] or with TOM (tri-iso-propylsilyloxymethyl) group,[38][39] both being removable by treatment with fluoride ion.
- The phosphite moiety also bears a diisopropylamino (iPr2N) group reactive under acidic conditions. On activation, the diisopropylamino group leaves, to be substituted by the 5'-hydroxy group of the support-bound oligonucleotide.
See also
- DNA synthesis
- Nucleic acid analogues
- Oligonucleotide synthesis
References
- Beaucage, S.L.; Caruthers M.H. (1981). "Deoxynucleoside phosphoramidites—A new class of key intermediates for deoxypolynucleotide synthesis". Tetrahedron Letters. 22: 1859–1862. doi:10.1016/S0040-4039(01)90461-7.
- Lin, K.-Y., Matteucci, M. D. (1998). "A cytosine analog capable of clamp-like binding to a guanine in helical nucleic acids". J. Am. Chem. Soc. 120 (33): 8531–8532. doi:10.1021/ja981286z.CS1 maint: multiple names: authors list (link)
- Nielsen, J.; Marugg, J. E.; Taagaard, M.; Van Boom, J. H.; Dahl, O. (1986). "Polymer-supported synthesis of deoxyoligonucleotides using in situ prepared deoxynucleoside 2-cyanoethyl phosphoramidites". Rec. Trav. Chim. Pays-Bas. 105 (1): 33–34.
- Nielsen, J.; Taagaard, M.; Marugg, J. E.; Van Boom, J. H.; Dahl, O. (1986). "Application of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite for in situ preparation of deoxyribonucleoside phosphoramidites and their use in polymer-supported synthesis of oligodeoxyribonucleotides". Nucleic Acids Res. 14 (18): 7391–7403. doi:10.1093/nar/14.18.7391. PMC 311758. PMID 3763407.
- Nielsen, J.; Marugg, J. E.; Van Boom, J. H.; Honnens, J.; Taagaard, M.; Dahl, O. (1986). "Thermal instability of some alkyl phosphorodiamidites". J. Chem Res. Synopses (1): 26–27.
- Nielsen, J.; Dahl, O. (1987). "Improved synthesis of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite (iPr2N)2POCH2CH2CN)". Nucleic Acids Res. 15 (8): 3626. doi:10.1093/nar/15.8.3626. PMC 340760. PMID 3575107.
- Beaucage, S. L. (2001). "2-Cyanoethyl Tetraisopropylphosphorodiamidite". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00312.
- Sinha, N. D.; Biernat, J.; Koester, H. (1983). "β-Cyanoethyl N,N-dialkylamino/N-morpholinomonochloro phosphoamidites, new phosphitylating agents facilitating ease of deprotection and work-up of synthesized oligonucleotides". Tetrahedron Lett. 24 (52): 5843–5846. doi:10.1016/S0040-4039(00)94216-3.
- Marugg, J. E.; Burik, A.; Tromp, M.; Van der Marel, G. A. & Van Boom, J. H. (1986). "A new and versatile approach to the preparation of valuable deoxynucleoside 3'-phosphite intermediates". Tetrahedron Lett. 24 (20): 2271–22274. doi:10.1016/S0040-4039(00)84506-2.
- Guzaev, A. P.; Manoharan, M. (2001). "2-Benzamidoethyl group - a novel type of phosphate protecting group for oligonucleotide synthesis". J. Am. Chem. Soc. 123 (5): 783–793. doi:10.1021/ja0016396.
- Sproat, B.; Colonna, F.; Mullah, B.; Tsou, D.; Andrus, A.; Hampel, A.; Vinayak, R. (Feb 1995). "An efficient method for the isolation and purification of oligoribonucleotides". Nucleosides & Nucleotides. 14 (1&2): 255–273. doi:10.1080/15257779508014668. ISSN 0261-3166.
- Stutz, A.; Hobartner, C.; Pitsch, S. (Sep 2000). "Novel fluoride-labile nucleobase-protecting groups for the synthesis of 3'(2')-O-amino-acylated RNA sequences". Helv. Chim. Acta. 83 (9): 2477–2503. doi:10.1002/1522-2675(20000906)83:9<2477::aid-hlca2477>3.0.co;2-9. ISSN 0018-019X.
- Welz, R.; Muller, S. (Jan 2002). "5-(Benzylmercapto)-1H-tetrazole as activator for 2'-O-TBDMS phosphoramidite building blocks in RNA synthesis". Tetrahedron Letters. 43 (5): 795–797. doi:10.1016/S0040-4039(01)02274-2. ISSN 0040-4039.
- Vargeese, C.; Carter, J.; Yegge, J.; Krivjansky, S.; Settle, A.; Kropp, E.; Peterson, K.; Pieken, W. (1998). "Efficient activation of nucleoside phosphoramidites with 4,5-dicyanoimidazole during oligonucleotide synthesis". Nucleic Acids Res. 26 (4): 1046–1050. doi:10.1093/nar/26.4.1046. ISSN 0305-1048. PMC 147346. PMID 9461466.
- Gacs-Baitz, E.; Sipos, F.; Egyed, O.; Sagi, G. (2009). "Synthesis and structural study of variously oxidized diastereomeric 5'-dimethoxytrityl-thymidine-3'-O-[O-(2-cyanoethyl)-N,N-diisopropyl]-phosphoramidite derivatives. Comparison of the effects of the P=O, P=S, and P=Se functions on the NMR spectral and chromatographic properties". Chirality. 21 (7): 663–673. doi:10.1002/chir.20653.
- Nemer, M. J.; Ogilvie, K. K. (1980). "Phosphoramidate analogs of diribonucleoside monophosphates". Tetrahedron Lett. 21 (43): 4153–4154. doi:10.1016/s0040-4039(00)93675-x.
- Wilk, A.; Uznanski, B.; Stec, W. J. (1991). "Assignment of absolute configuration at phosphorus in dithymidylyl(3',5')phosphormorpholidates and -phosphormorpholidothioates". Nucleosides & Nucleotides. 10 (1–3): 319–322. doi:10.1080/07328319108046469.
- Guzaev, A. P. (2011). "Reactivity of 3H-1,2,4-dithiazole-3-thiones and 3H-1,2-dithiole-3-thiones as sulfurizing agents for oligonucleotide synthesis". Tetrahedron Letters. 52: 434–437. doi:10.1016/j.tetlet.2010.11.086.
- Holloway, G. A.; Pavot, C.; Scaringe, S. A.; Lu, Y.; Rauchfuss, T. B. (2002). "An organometallic route to oligonucleotides containing phosphoroselenoate". ChemBioChem. 3 (11): 1061–1065. doi:10.1002/1439-7633(20021104)3:11<1061::aid-cbic1061>3.0.co;2-9.
- Ravikumar, V. T.; Kumar, R. K. (2004). "Stereoselective Synthesis of Alkylphosphonates: A Facile Rearrangement of Cyanoethyl-Protected Nucleoside Phosphoramidites". Org. Process Res. Dev. 8 (4): 603–608. doi:10.1021/op030035u.
- "Beta-Cyanoethyl Phosphoramidites". Products.appliedbiosystems.com. Retrieved 2009-05-12.
- "Biosearch Technologies". Biosearchtech.com. Retrieved 2009-05-12.
- "ChemGenes Corporation, a Biotechnology company". Chemgenes.com. Retrieved 2009-05-12.
- M. Powell (2008-01-17). "Applied Biosystems Instruments". Glenresearch.com. Retrieved 2009-05-12.
- "Nucleic Acid Synthesis & Labeling". Thermo.com. 2008-08-16. Archived from the original on February 28, 2009. Retrieved 2009-05-12.
- Gryaznov, S. M.; Letsinger, R. L. (1991). "Synthesis of oligonucleotides via monomers with unprotected bases". J. Am. Chem. Soc. 113 (15): 5876–5877. doi:10.1021/ja00015a059.
- Reddy, M. P.; Hanna, N. B.; Farooqui, F. (1997). "Ultrafast Cleavage and Deprotection of Oligonucleotides Synthesis and Use of CAc Derivatives". Nucleosides & Nucleotides. 16: 1589–1598. doi:10.1080/07328319708006236.
- McMinn, D. (1997). "Synthesis of oligonucleotides containing 3'-alkyl amines using N-isobutyryl protected deoxyadenosine phosphoramidite". Tetrahedron Lett. 38: 3123. doi:10.1016/S0040-4039(97)00568-6.
- Schulhof, J. C.; Molko, D.; Teoule, R. (1987). "The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups". Nucleic Acids Res. 15 (2): 397–416. doi:10.1093/nar/15.2.397. PMC 340442. PMID 3822812.
- Zhu, Q. (2001). "Observation and elimination of N-acetylation of oligonucleotides prepared using fast-deprotecting phosphoramidites and ultra-mild deprotection". Bioorg. Med. Chem. Lett. 11: 1105. doi:10.1016/S0960-894X(01)00161-5. PMID 11354354.
- McBride, L. J.; Kierzek, R.; Beaucage, S. L.; Caruthers, M. H. (1986). "Nucleotide chemistry. 16. Amidine protecting groups for oligonucleotide synthesis". J. Am. Chem. Soc. 108: 2040. doi:10.1021/ja00268a052.
- Sinha, N. D.; Biernat, J.; McManus, J.; Koester, H. (1984). "Polymer support oligonucleotide synthesis. XVIII: use of β-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product". Nucleic Acids Res. 12 (11): 4539–4557. doi:10.1093/nar/12.11.4539. PMC 318857. PMID 6547529.
- Guzaev, A. P.; Manoharan, M. (2001). "Phosphoramidite Coupling to Oligonucleotides Bearing Unprotected Internucleosidic Phosphate Moieties". J. Org. Chem. 66 (5): 1798–1804. doi:10.1021/jo001591e. PMID 11262130.
- Ogilvie, K. K.; Theriault, N.; Sadana, K. L. (1977). "Synthesis of oligoribonucleotides". J. Am. Chem. Soc. 99 (23): 7741–7743. doi:10.1021/ja00465a073. PMID 915168.
- Usman, N.; Ogilvie, K. K.; Jiang, M. Y.; Cedergren, R. J. (1987). "The automated chemical synthesis of long oligoribuncleotides using 2'-O-silylated ribonucleoside 3'-O-phosphoramidites on a controlled-pore glass support: synthesis of a 43-nucleotide sequence similar to the 3'-half molecule of an Escherichia coli formylmethionine tRNA". J. Am. Chem. Soc. 109 (25): 7845–7854. doi:10.1021/ja00259a037.
- Usman, N.; Pon, R. T.; Ogilvie, K. K. (1985). "Preparation of ribonucleoside 3'-O-phosphoramidites and their application to the automated solid phase synthesis of oligonucleotides". Tetrahedron Lett. 26 (38): 4567–4570. doi:10.1016/S0040-4039(00)98753-7.
- Scaringe, S. A.; Francklyn, C.; Usman, N. (1990). "Chemical synthesis of biologically active oligoribonucleotides using β-cyanoethyl protected ribonucleoside phosphoramidites". Nucleic Acids Res. 18 (18): 5433–5441. doi:10.1093/nar/18.18.5433. PMC 332221. PMID 2216717.
- Pitsch, S.; Weiss, P. A.; Wu, X.; Ackermann, D.; Honegger, T. (1999). "Fast and reliable automated synthesis of RNA and partially 2'-O-protected precursors ("caged RNA") based on two novel, orthogonal 2'-O-protecting groups". Helv. Chim. Acta. 82 (10): 1753–1761. doi:10.1002/(SICI)1522-2675(19991006)82:10<1753::AID-HLCA1753>3.0.CO;2-Y.
- Pitsch, S.; Weiss, P. A.; Jenny, L.; Stutz, A.; Wu, X. (2001). "Reliable chemical synthesis of oligoribonucleotides (RNA) with 2'-O-[(triisopropylsilyl)oxy]methyl(2'-O-tom)-protected phosphoramidites". Helv. Chim. Acta. 84 (12): 3773–3795. doi:10.1002/1522-2675(20011219)84:12<3773::AID-HLCA3773>3.0.CO;2-E.
Further reading
- Comprehensive Natural Products Chemistry, Volume 7: DNA and Aspects of Molecular Biology. Kool, Eric T.; Editor. Neth. (1999), 733 pp. Publisher: (Elsevier, Amsterdam, Neth.)
- Beaucage S. L., Iyer R. P. (1992). "Advances in the synthesis of oligonucleotides by the phosphoramidite approach". Tetrahedron. 48: 2223–2311. doi:10.1016/s0040-4020(01)88752-4.
- Beaucage S. L., Iyer R. P. (1993). "The functionalization of oligonucleotides via phosphoramidite derivatives". Tetrahedron. 49: 1925–1963. doi:10.1016/s0040-4020(01)86295-5.
- Beaucage S. L., Iyer R. P. (1993). "The synthesis of modified oligonucleotides by the phosphoramidite approach and their applications". Tetrahedron. 49: 6123–6194. doi:10.1016/s0040-4020(01)87958-8.
- Beaucage, S L. "Oligodeoxyribonucleotides synthesis. Phosphoramidite approach. Methods in Molecular Biology (Totowa, NJ, United States) (1993), 20 (Protocols for Oligonucleotides and Analogs), 33–61.
- Reese C. B. (2002). "The chemical synthesis of oligo- and poly-nucleotides: a personal commentary". Tetrahedron. 58: 8893–8920. doi:10.1016/s0040-4020(02)01084-0.
- Brown T., Brown D. J. S. 1991. In Oligonucleotides and Analogues. A Practical Approach, ed. F Eckstein, pp. 1 – 24. Oxford: IRL