Redox
Redox (reduction–oxidation, pronunciation: /ˈrɛdɒks/ redoks or /ˈriːdɒks/ reedoks[1]) is a type of chemical reaction in which the oxidation states of atoms are changed. Redox reactions are characterized by the actual or formal transfer of electrons between chemical species, most often with one species (the reducing agent) undergoing oxidation (losing electrons) while another species (the oxidizing agent) undergoes reduction (gains electrons).[2] The chemical species from which the electron is removed is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. In other words:
- Oxidation is the loss of electrons or an increase in the oxidation state of an atom, an ion, or of certain atoms in a molecule.
- Reduction is the gain of electrons or a decrease in the oxidation state of an atom, an ion, or of certain atoms in a molecule.
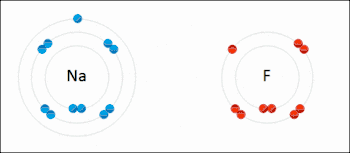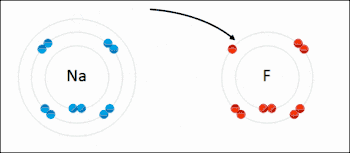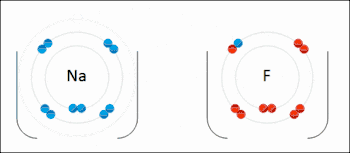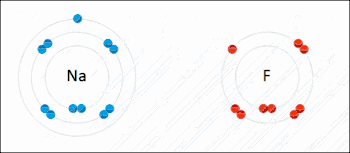
Many reactions in organic chemistry are redox reactions due to changes in oxidation states but without distinct electron transfer. For example, during the combustion of wood with molecular oxygen, the oxidation state of carbon atoms in the wood increases and that of oxygen atoms decreases as carbon dioxide and water are formed. The oxygen atoms undergo reduction, formally gaining electrons, while the carbon atoms undergo oxidation, losing electrons. Thus oxygen is the oxidizing agent and carbon is the reducing agent in this reaction.[3]
Although oxidation reactions are commonly associated with the formation of oxides from oxygen molecules, oxygen is not necessarily included in such reactions, as other chemical species can serve the same function.[3]
Redox reactions can occur relatively slowly, as in the formation of rust, or much more rapidly, as in the case of burning fuel. There are simple redox processes, such as the oxidation of carbon to yield carbon dioxide (CO2) or the reduction of carbon by hydrogen to yield methane (CH4), and more complex processes such as the oxidation of glucose (C6H12O6) in the human body. Analysis of bond energies and ionization energies in water allow calculation of the redox potentials.[4][5]
Etymology
"Redox" is a portmanteau of the words "reduction" and "oxidation". The word oxidation originally implied reaction with oxygen to form an oxide, since dioxygen (O2(g)) was historically the first recognized oxidizing agent. Later, the term was expanded to encompass oxygen-like substances that accomplished parallel chemical reactions. Ultimately, the meaning was generalized to include all processes involving loss of electrons.
The word reduction originally referred to the loss in weight upon heating a metallic ore such as a metal oxide to extract the metal. In other words, ore was "reduced" to metal. Antoine Lavoisier showed that this loss of weight was due to the loss of oxygen as a gas. Later, scientists realized that the metal atom gains electrons in this process. The meaning of reduction then became generalized to include all processes involving a gain of electrons.
The electrochemist John Bockris has used the words electronation and deelectronation to describe reduction and oxidation processes, respectively, when they occur at electrodes.[6] These words are analogous to protonation and deprotonation,[7] but they have not been widely adopted by chemists worldwide.
The term "hydrogenation" could often be used instead of reduction, since hydrogen is the reducing agent in a large number of reactions, especially in organic chemistry and biochemistry. But, unlike oxidation, which has been generalized beyond its root element, hydrogenation has maintained its specific connection to reactions that add hydrogen to another substance (e.g., the hydrogenation of unsaturated fats into saturated fats, R−CH=CH−R + H2 → R−CH2−CH2−R). The word "redox" was first used in 1928.[8]
Definitions
The processes of oxidation and reduction occur simultaneously and cannot happen independently of one another, similar to acid–base reactions.[3] The oxidation alone and the reduction alone are each called a half-reaction, because two half-reactions always occur together to form a whole reaction. When writing half-reactions, the gained or lost electrons are typically included explicitly in order that the half-reaction be balanced with respect to electric charge. The electrons cancel out when the half-reactions are combined to make the net chemical equation.
Though sufficient for many purposes, these general descriptions are not precisely correct. Although oxidation and reduction properly refer to a change in oxidation state, the actual transfer of electrons may never occur. The oxidation state of an atom is the fictitious charge that an atom would have if all bonds between atoms of different elements were 100% ionic. Thus, oxidation is best defined as an increase in oxidation state, and reduction as a decrease in oxidation state. In practice, the transfer of electrons will always cause a change in oxidation state, but there are many reactions that are classed as "redox" even though no electron transfer occurs (such as those involving covalent bonds). As a result, simple half-reactions cannot be written for the individual atoms undergoing a redox process.
Oxidizing and reducing agents
In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or reducing agent loses electrons and is oxidized, and the oxidant or oxidizing agent gains electrons and is reduced. The pair of an oxidizing and reducing agent that are involved in a particular reaction is called a redox pair. A redox couple is a reducing species and its corresponding oxidizing form, e.g., Fe2+
/Fe3+
Oxidizers
Substances that have the ability to oxidize other substances (cause them to lose electrons) are said to be oxidative or oxidizing and are known as oxidizing agents, oxidants, or oxidizers. That is, the oxidant (oxidizing agent) removes electrons from another substance, and is thus itself reduced. And, because it "accepts" electrons, the oxidizing agent is also called an electron acceptor. Oxygen is the quintessential oxidizer.
Oxidants are usually chemical substances with elements in high oxidation states (e.g., H
2O
2, MnO−
4, CrO
3, Cr
2O2−
7, OsO
4), or else highly electronegative elements (O2, F2, Cl2, Br2) that can gain extra electrons by oxidizing another substance.
Reducers
Substances that have the ability to reduce other substances (cause them to gain electrons) are said to be reductive or reducing and are known as reducing agents, reductants, or reducers. The reductant (reducing agent) transfers electrons to another substance, and is thus itself oxidized. And, because it donates electrons, the reducing agent is also called an electron donor. Electron donors can also form charge transfer complexes with electron acceptors.
Reductants in chemistry are very diverse. Electropositive elemental metals, such as lithium, sodium, magnesium, iron, zinc, and aluminium, are good reducing agents. These metals donate or give away electrons relatively readily. Hydride transfer reagents, such as NaBH4 and LiAlH4, are widely used in organic chemistry,[9][10] primarily in the reduction of carbonyl compounds to alcohols. Another method of reduction involves the use of hydrogen gas (H2) with a palladium, platinum, or nickel catalyst. These catalytic reductions are used primarily in the reduction of carbon-carbon double or triple bonds.
Standard electrode potentials (reduction potentials)
Each half-reaction has a standard electrode potential (E0
cell), which is equal to the potential difference or voltage at equilibrium under standard conditions of an electrochemical cell in which the cathode reaction is the half-reaction considered, and the anode is a standard hydrogen electrode where hydrogen is oxidized:
- 1⁄2 H2 → H+ + e−.
The electrode potential of each half-reaction is also known as its reduction potential E0
red, or potential when the half-reaction takes place at a cathode. The reduction potential is a measure of the tendency of the oxidizing agent to be reduced. Its value is zero for H+ + e− → 1⁄2 H2 by definition, positive for oxidizing agents stronger than H+ (e.g., +2.866 V for F2) and negative for oxidizing agents that are weaker than H+ (e.g., −0.763 V for Zn2+).[11]
For a redox reaction that takes place in a cell, the potential difference is:
- E0
cell = E0
cathode – E0
anode
However, the potential of the reaction at the anode was sometimes expressed as an oxidation potential:
- E0
ox = –E0
red.
The oxidation potential is a measure of the tendency of the reducing agent to be oxidized, but does not represent the physical potential at an electrode. With this notation, the cell voltage equation is written with a plus sign
- E0
cell = E0
red(cathode) + E0
ox(anode)
Examples of redox reactions
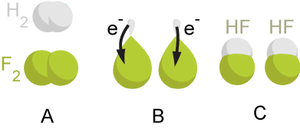
In the reaction between hydrogen and fluorine, hydrogen is being oxidized and fluorine is being reduced:
- H
2 + F
2 → 2 HF
This reaction is spontaneous and releases 542 kJ per 2 g of hydrogen because the H-F bond is much stronger than the weak, high-energy F-F bond. We can write this overall reaction as two half-reactions:
the oxidation reaction:
and the reduction reaction:
- F
2 + 2 e− → 2 F−
Analyzing each half-reaction in isolation can often make the overall chemical process clearer. Because there is no net change in charge during a redox reaction, the number of electrons in excess in the oxidation reaction must equal the number consumed by the reduction reaction (as shown above).
Elements, even in molecular form, always have an oxidation state of zero. In the first half-reaction, hydrogen is oxidized from an oxidation state of zero to an oxidation state of +1. In the second half-reaction, fluorine is reduced from an oxidation state of zero to an oxidation state of −1.
When adding the reactions together the electrons are canceled:
H
2→ 2 H+ + 2 e− F
2 + 2 e−→ 2 F− H2 + F2 → 2 H+ + 2 F−
And the ions combine to form hydrogen fluoride:
- 2 H+ + 2 F− → 2 HF
The overall reaction is:
- H
2 + F
2 → 2 HF
Metal displacement
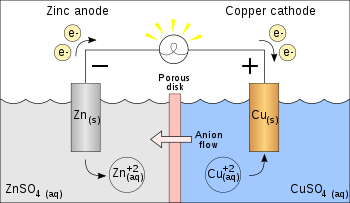
In this type of reaction, a metal atom in a compound (or in a solution) is replaced by an atom of another metal. For example, copper is deposited when zinc metal is placed in a copper(II) sulfate solution:
Zn(s)+ CuSO4(aq) → ZnSO4(aq) + Cu(s)
In the above reaction, zinc metal displaces the copper(II) ion from copper sulfate solution and thus liberates free copper metal. The reaction is spontaneous and releases 213 kJ per 65 g of zinc because relative to zinc, copper metal is lower in energy due to bonding via its partially filled d-orbitals.[4]
The ionic equation for this reaction is:
- Zn + Cu2+ → Zn2+ + Cu
As two half-reactions, it is seen that the zinc is oxidized:
- Zn → Zn2+ + 2 e−
And the copper is reduced:
- Cu2+ + 2 e− → Cu
Other examples
- The reduction of nitrate to nitrogen in the presence of an acid (denitrification):
- 2 NO−
3 + 10 e− + 12 H+ → N2 + 6 H2O
- 2 NO−
- The combustion of hydrocarbons, such as in an internal combustion engine, produces water, carbon dioxide, some partially oxidized forms such as carbon monoxide, and heat energy. Complete oxidation of materials containing carbon produces carbon dioxide.
- In organic chemistry, the stepwise oxidation of a hydrocarbon by oxygen produces water and, successively, an alcohol, an aldehyde or a ketone, a carboxylic acid, and then a peroxide.
Corrosion and rusting

- The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion; it forms as a result of the oxidation of iron metal. Common rust often refers to iron(III) oxide, formed in the following chemical reaction:
- 4 Fe + 3 O2 → 2 Fe2O3
- The oxidation of iron(II) to iron(III) by hydrogen peroxide in the presence of an acid:
- Fe2+ → Fe3+ + e−
- H2O2 + 2 e− → 2 OH−
- Overall equation:
- 2 Fe2+ + H2O2 + 2 H+ → 2 Fe3+ + 2 H2O
Disproportionation
A disproportionation reaction is one in which a single substance is both oxidized and reduced. For example, thiosulfate ion with sulfur in oxidation state +2 can react in the presence of acid to form elemental sulfur (oxidation state 0) and sulfur dioxide (oxidation state +4).
- S2O32-(aq) + 2 H+(aq) → S(s) + SO2(g) + H2O(l)
Thus one sulfur atom is reduced from +2 to 0, while the other is oxidized from +2 to +4.[12]
Redox reactions in industry
Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects protected metal to a more easily corroded "sacrificial anode" to act as the anode. The sacrificial metal instead of the protected metal, then, corrodes. A common application of cathodic protection is in galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.
Oxidation is used in a wide variety of industries such as in the production of cleaning products and oxidizing ammonia to produce nitric acid, which is used in most fertilizers.
Redox reactions are the foundation of electrochemical cells, which can generate electrical energy or support electrosynthesis. Metal ores often contain metals in oxidized states such as oxides or sulfides, from which the pure metals are extracted by smelting at high temperature in the presence of a reducing agent. The process of electroplating uses redox reactions to coat objects with a thin layer of a material, as in chrome-plated automotive parts, silver plating cutlery, galvanization and gold-plated jewelry.
Redox reactions in biology
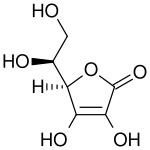 |
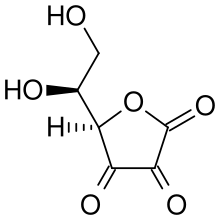 |
Bottom: dehydroascorbic acid (oxidized form of Vitamin C)
Many important biological processes involve redox reactions.
Cellular respiration, for instance, is the oxidation of glucose (C6H12O6) to CO2 and the reduction of oxygen to water. The summary equation for cell respiration is:
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
The process of cell respiration also depends heavily on the reduction of NAD+ to NADH and the reverse reaction (the oxidation of NADH to NAD+). Photosynthesis and cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
- 6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2
Biological energy is frequently stored and released by means of redox reactions. Photosynthesis involves the reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reduce nicotinamide adenine dinucleotide (NAD+) to NADH, which then contributes to the creation of a proton gradient, which drives the synthesis of adenosine triphosphate (ATP) and is maintained by the reduction of oxygen. In animal cells, mitochondria perform similar functions. See the Membrane potential article.
Free radical reactions are redox reactions that occur as a part of homeostasis and killing microorganisms, where an electron detaches from a molecule and then reattaches almost instantaneously. Free radicals are a part of redox molecules and can become harmful to the human body if they do not reattach to the redox molecule or an antioxidant. Unsatisfied free radicals can spur the mutation of cells they encounter and are, thus, causes of cancer.
The term redox state is often used to describe the balance of GSH/GSSG, NAD+/NADH and NADP+/NADPH in a biological system such as a cell or organ. The redox state is reflected in the balance of several sets of metabolites (e.g., lactate and pyruvate, beta-hydroxybutyrate, and acetoacetate), whose interconversion is dependent on these ratios. An abnormal redox state can develop in a variety of deleterious situations, such as hypoxia, shock, and sepsis. Redox mechanism also control some cellular processes. Redox proteins and their genes must be co-located for redox regulation according to the CoRR hypothesis for the function of DNA in mitochondria and chloroplasts.
Redox cycling
A wide variety of aromatic compounds are enzymatically reduced to form free radicals that contain one more electron than their parent compounds. In general, the electron donor is any of a wide variety of flavoenzymes and their coenzymes. Once formed, these anion free radicals reduce molecular oxygen to superoxide, and regenerate the unchanged parent compound. The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide. This catalytic behavior has been described as a futile cycle or redox cycling.
Redox reactions in geology
In geology, redox is important to both the formation of minerals and the mobilization of minerals, and is also important in some depositional environments. In general, the redox state of most rocks can be seen in the color of the rock. The rock forms in oxidizing conditions, giving it a red color. It is then "bleached" to a green—or sometimes white—form when a reducing fluid passes through the rock. The reduced fluid can also carry uranium-bearing minerals. Famous examples of redox conditions affecting geological processes include uranium deposits and Moqui marbles.
Balancing redox reactions
Describing the overall electrochemical reaction for a redox process requires a balancing of the component half-reactions for oxidation and reduction. In general, for reactions in aqueous solution, this involves adding H+, OH−, H2O, and electrons to compensate for the oxidation changes.
Acidic media
In acidic aqueous media, H+ ions and water are added to half-reactions to balance the overall reaction.
For instance, when manganese(II) reacts with sodium bismuthate:
Unbalanced reaction: Mn2+(aq) + NaBiO3(s) → Bi3+(aq) + MnO−
4 (aq)Oxidation: 4 H2O(l) + Mn2+(aq) → MnO−
4(aq) + 8 H+(aq) + 5 e−Reduction: 2 e− + 6 H+ + BiO−
3(s) → Bi3+(aq) + 3 H2O(l)
The reaction is balanced by scaling the two half-cell reactions to involve the same number of electrons (multiplying the oxidation reaction by the number of electrons in the reduction step and vice versa):
- 8 H2O(l) + 2 Mn2+(aq) → 2 MnO−
4(aq) + 16 H+(aq) + 10 e− - 10 e− + 30 H+ + 5 BiO−
3(s) → 5 Bi3+(aq) + 15 H2O(l)
Adding these two reactions eliminates the electrons terms and yields the balanced reaction:
- 14 H+(aq) + 2 Mn2+(aq) + 5 NaBiO3(s) → 7 H2O(l) + 2 MnO−
4(aq) + 5 Bi3+(aq) + 5 Na+
(aq)
Basic media
In basic aqueous media, OH− ions and water are added to half reactions to balance the overall reaction.
For example, in the reaction between potassium permanganate and sodium sulfite:
Unbalanced reaction: KMnO4 + Na2SO3 + H2O → MnO2 + Na2SO4 + KOH Reduction: 3 e− + 2 H2O + MnO−
4 → MnO2 + 4 OH−Oxidation: 2 OH− + SO2−
3 → SO2−
4 + H2O + 2 e−
Balancing the number of electrons in the two half-cell reactions gives:
- 6 e− + 4 H2O + 2 MnO−
4 → 2 MnO2 + 8 OH− - 6 OH− + 3 SO2−
3 → 3 SO2−
4 + 3 H2O + 6 e−
Adding these two half-cell reactions together gives the balanced equation:
- 2 KMnO4 + 3 Na2SO3 + H2O → 2 MnO2 + 3 Na2SO4 + 2 KOH
Mnemonics
The key terms involved in redox are often confusing.[13][14] For example, a reagent that is oxidized loses electrons; however, that reagent is referred to as the reducing agent. Likewise, a reagent that is reduced gains electrons and is referred to as the oxidizing agent.[15] These mnemonics are commonly used by students to help memorise the terminology:[16]
- "OIL RIG" — oxidation is loss of electrons, reduction is gain of electrons[13][14][15][16]
- "LEO the lion says GER" — loss of electrons is oxidation, gain of electrons is reduction[13][14][15][16]
- "LEORA says GEROA" — loss of electrons is oxidation (reducing agent), gain of electrons is reduction (oxidizing agent).[15]
- "RED CAT" and "AN OX", or "AnOx RedCat" ("an ox-red cat") — reduction occurs at the cathode and the anode is for oxidation
- "RED CAT gains what AN OX loses" – reduction at the cathode gains (electrons) what anode oxidation loses (electrons)
- "PANIC" – Positive Anode and Negative is Cathode. This applies to electrolytic cells which release stored electricity, and can be recharged with electricity. PANIC does not apply to cells that can be recharged with redox materials. These galvanic or voltaic cells, such as fuel cells, produce electricity from internal redox reactions. In these the positive electrode is the cathode and the negative is the anode.
See also
- Anaerobic respiration
- Bessemer process
- Bioremediation
- Calvin cycle
- Chemical equation
- Chemical looping combustion
- Citric acid cycle
- Electrochemical series
- Electrochemistry
- Electrolysis
- Electron equivalent
- Electron transport chain
- Electrosynthesis
- Galvanic cell
- Hydrogenation
- Membrane potential
- Microbial fuel cell
- Nucleophilic abstraction
- Organic redox reaction
- Oxidative addition and reductive elimination
- Oxidative phosphorylation
- Partial oxidation
- Pro-oxidant
- Reduced gas
- Reducing agent
- Reducing atmosphere
- Reduction potential
- Thermic reaction
- Transmetalation
- Sulfur Cycle
References
- "redox – definition of redox in English | Oxford Dictionaries". Oxford Dictionaries | English. Archived from the original on 2017-10-01. Retrieved 2017-05-15.
- "Redox Reactions". wiley.com. Archived from the original on 2012-05-30. Retrieved 2012-05-09.
- Haustein, Catherine Hinga (2014). "Oxidation–reduction reaction". In K. Lee Lerner; Brenda Wilmoth Lerner (eds.). The Gale Encyclopedia of Science (5th ed.). Farmington Hills, MI: Gale Group.
- Schmidt-Rohr, K. (2018). "How Batteries Store and Release Energy: Explaining Basic Electrochemistry". J. Chem. Educ. 95 (10): 1801–1810. doi:10.1021/acs.jchemed.8b00479.
- Schmidt-Rohr, K. (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2". J. Chem. Educ. 92 (12): 2094–2099. doi:10.1021/acs.jchemed.5b00333.
- Bockris, John O'M.; Reddy, Amulya K. N. (1970). Modern Electrochemistry. Plenum Press. pp. 352–3.
- Bockris, John O'M.; Reddy, Amulya K.N. (2013) [1970]. Modern Electrochemistry. Volume 1. Springer Science & Business Media. p. 494. ISBN 9781461574675. Retrieved 29 March 2020.
The homogeneous proton-transfer reactions described are similar to homogeneous electron-transfer reactions in that the overall electron-transfer reaction can be decomposed into one electronation reaction and one deelectronation reaction.
- Harper, Douglas. "redox". Online Etymology Dictionary.
- Hudlický, Miloš (1996). Reductions in Organic Chemistry. Washington, D.C.: American Chemical Society. p. 429. ISBN 978-0-8412-3344-7.
- Hudlický, Miloš (1990). Oxidations in Organic Chemistry. Washington, D.C.: American Chemical Society. pp. 456. ISBN 978-0-8412-1780-5.
- Electrode potential values from: Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General chemistry: principles and modern applications (8th ed.). Upper Saddle River, N.J: Prentice Hall. p. 832. ISBN 978-0-13-014329-7. LCCN 2001032331. OCLC 46872308.CS1 maint: ref=harv (link)
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry. Principles and Modern Applications (8th ed.). Prentice Hall. p. 158. ISBN 0-13-014329-4.
- Robertson, William (2010). More Chemistry Basics. National Science Teachers Association. p. 82. ISBN 978-1-936137-74-9.
- Phillips, John; Strozak, Victor; Wistrom, Cheryl (2000). Chemistry: Concepts and Applications. Glencoe McGraw-Hill. p. 558. ISBN 978-0-02-828210-7.
- Rodgers, Glen (2012). Descriptive Inorganic, Coordination, and Solid-State Chemistry. Brooks/Cole, Cengage Learning. p. 330. ISBN 978-0-8400-6846-0.
- Zumdahl, Steven; Zumdahl, Susan (2009). Chemistry. Houghton Mifflin. p. 160. ISBN 978-0-547-05405-6.
Further reading
- Schüring, J.; Schulz, H. D.; Fischer, W. R.; Böttcher, J.; Duijnisveld, W. H., eds. (1999). Redox: Fundamentals, Processes and Applications. Heidelberg: Springer-Verlag. p. 246. hdl:10013/epic.31694.d001. ISBN 978-3-540-66528-1.CS1 maint: ref=harv (link)
- Tratnyek, Paul G.; Grundl, Timothy J.; Haderlein, Stefan B., eds. (2011). Aquatic Redox Chemistry. ACS Symposium Series. 1071. doi:10.1021/bk-2011-1071. ISBN 978-0-8412-2652-4.CS1 maint: ref=harv (link)
External links
| Wikiquote has quotations related to: Redox |
| Wikimedia Commons has media related to Redox reactions. |
- Chemical Equation Balancer – An open source chemical equation balancer that handles redox reactions.
- Redox reactions calculator
- Redox reactions at Chemguide
- Online redox reaction equation balancer, balances equations of any half-cell and full reactions