Pentacarbonylhydridomanganese
Pentacarbonylhydridomanganese is an organometallic compound with formula HMn(CO)5. This compound is one of the most stable "first-row" transition metal hydrides.
5-2D.png) | |
 | |
| Names | |
|---|---|
| Other names
Hydrogen pentacarbonylmanganate(-I) (7CI); Manganese, pentacarbonylhydro- (8CI); Hydridomanganese pentacarbonyl; Hydridopentacarbonylmanganese; Manganese pentacarbonyl hydride; Pentacarbonylhydromanganese; Pentacarbonylmanganese hydride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| HMn(CO)5 | |
| Molar mass | 195.99799 g/mol |
| Appearance | At room temperature, it is liquid and colorless. Below its melting point, it may be sublimed in vacuum.[1] |
| Acidity (pKa) | 7.1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
It was first reported in 1931.[2] Of the several ways to produce this compound,[3] is the protonation of the pentacarbonyl manganate anion. The latter is formed from reduction of dimanganese decacarbonyl, (Mn(CO)5)2. The reaction is shown below.
- LiHB(C2H5)3 (Superhydride) + ½ Mn2(CO)10 → Li[Mn(CO)5] + ½ H2 + (C2H5)3B
- Li[Mn(CO)5] + CF3SO3H → HMn(CO)5 + Li+
CF3SO3−
Salts of [Mn(CO)5]−
can be isolated as crystalline PPN+
(μ-nitrido—bis-(triphenylphosphorus)) salt, which is smoothly protonated by CF3SO3H.[3]
- PPN[Mn(CO)5] + CF3SO3H → HMn(CO)5 + PPN+
CF3SO3−
This compound can also be formed by the reaction of a solution of pentacarbonyl(trimethylsilyl)manganese with water.[4] The reaction is shown below.
- 2 (CO)5MnSiMe3 + H2O → HMn(CO)5 + Me3SiOSiMe3
Structure and properties
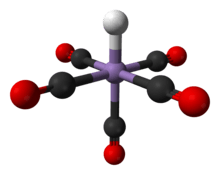
The compound has octahedral symmetry [5] and its molecular point group is C4v.[6] The H-Mn bond length is 1.44 ± 0.03 Å.[6] A gas phase electron diffraction study confirms this data.
The structure of HMn(CO)5 has been studied by many methods including X-ray diffraction, neutron diffraction, and electron diffraction.[6] HMn(CO)5 can be related to the structure of a hexacarbonyl complex such as Mn(CO)+
6, and therefore has the following similar properties.[7] The occupied molecular orbitals on the top are the 2 t2g orbitals. They are characterized as metal 3dπ orbitals. Since the antibonding 2π orbitals interact with the carbonyl groups, (or in this case, H−
) the t2g orbital is stabilized compared to the 3dπ orbital, which in turn will cause changes in the sigma and pi interactions.
Main reactions
The pKa of HMn(CO)5 in water is 7.1.[8] It is thus comparable to hydrogen sulfide, a common inorganic acid, in its acidity.
A common reaction involving the HMn(CO)5 species is substitution of the CO ligands by organophosphines, as occurs both thermally and photochemically.[9] In this way the following derivatives form MnH(CO)3P2, MnH(CO)2P3, and MnH(CO)P4, (where P = P(OEt)3, PPh(OEt)2, PPh2OEt, PPh(OiPr)2).
The compound HMn(CO)5 can be used to reduce olefins and other organic compounds, as well as metal halides.[3]
This compound can be methylated with diazomethane.[1]
- HMn(CO)5 + CH2N2 → Mn(CO)5CH3 + N2
Notes
- Eley, D.D.; Pines, Herman; Weisz, P.B. Advances In Catalysis. 32. 385. ISBN 978-0-12-007832-5
- Hieber, W. Leutert, F. Naturwissenschaften. 1931. 360.
- Hunter, Alan D; Bianconi, Larry J; DiMuzio, Steven J; Braho, Dianne L. Synthesis and Structure- Property Relationships in η6-Arene) Cr(CO)3 Chemistry: From Guided Experiments to Discovery Research. J. Chem. Educ. 75. 1998. 891. doi:10.1021/ed075p891
- Finn, M.G. Pentacarbonyl(trimethylsilyl)manganese. Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rp022s
- Liu, Xian-mei; Wang, Chao-yang; Qian-shu; Xie; Yaoming; King, R. Bruce; Schaefer, Henry F., III. Mononuclear and binuclear manganese carbonyl hydrides. Dalton Trans., 2009, 3774-3785, doi:10.1039/b822913a
- Kukolich, S.G. Microwave Spectrum and Molecular Structure for Manganese Pentacarbonyl Hydride. 33. 1994. 1217-1219
- Fenske, Richard. Electronic Structure and Bonding in Manganese Pentacarbonyl Halides and Hydride. Inorganic Chemistry. 9. 1970. 1053-1060.
- Morris, Robert H. (2016-08-10). "Brønsted–Lowry Acid Strength of Metal Hydride and Dihydrogen Complexes". Chemical Reviews. 116 (15): 8588–8654. doi:10.1021/acs.chemrev.5b00695. hdl:1807/78047. ISSN 0009-2665. PMID 26963836.
- Albertin, Gabriele. Cationic Molecular Hydrogen Complexes of Mn (I). Organometallics. 16. 1997. 4959-4969.