Manganocene
Manganocene or bis(cyclopentadienyl)manganese(II) is an organomanganese compound with the formula [Mn(C5H5)2]n. It is a thermochromic solid that degrades rapidly in air. Although the compound is of little utility, it is often discussed as an example of a metallocene with ionic character.[2]
 | |
| Names | |
|---|---|
| Other names
Bis(cyclopentadienyl)manganese | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.149.777 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H10Mn | |
| Molar mass | 185.128 g·mol−1 |
| Appearance | amber solid < 159 °C, pink > 159 °C |
| Melting point | 175 °C (347 °F; 448 K) |
| Boiling point | 245 °C (473 °F; 518 K) |
| Hazards[1] | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H228, H315, H319, H335 |
| P210, P261, P305+351+338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 52 °C (126 °F; 325 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and structure
It may be prepared in the manner common for other metallocenes, i.e., by reaction of manganese(II) chloride with sodium cyclopentadienide:
- MnCl2 + 2 CpNa → Cp2Mn + 2 NaCl
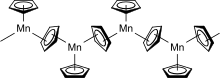
In the solid state below 159 °C, manganocene adopts a polymeric structure with every manganese atom coordinated by three cyclopentadienyl ligands, two of which are bridging ligands. Above 159 °C, the solid changes color from amber to pink and the polymer converts to the structure of a normal sandwich complex, i.e., the molecule Mn(η5-C5H5)2.[2]
Reactions
The ionic character of manganocene gives it an unusual pattern of reactivities compared to metallocenes of other transition metals in the same row. It is kinetically labile, being readily hydrolysed by water or hydrochloric acid, and readily forms adducts with two- or four-electron Lewis bases.[2]
Manganocene polymerizes ethylene to high molecular weight linear polyethylene in the presence of methylaluminoxane or diethylaluminium chloride as cocatalysts. It does not polymerize propylene.[2]
References
- "Bis(cyclopentadienyl)manganese". American Elements. Retrieved October 31, 2019.
- Layfield, Richard A. (2008). "Manganese(II): the black sheep of the organometallic family". Chem. Soc. Rev. 37: 1098–1107. doi:10.1039/B708850G.
