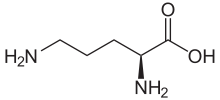
Ornithine
Ornithine is a non-proteinogenic amino acid that plays a role in the urea cycle. Ornithine is abnormally accumulated in the body in ornithine transcarbamylase deficiency. The radical is ornithyl.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
L-Ornithine | |
| Other names
(+)-(S)-2,5-Diaminovaleric acid (+)-(S)-2,5-Diaminopentanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.665 |
| EC Number |
|
| KEGG | |
| MeSH | Ornithine |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C5H12N2O2 | |
| Molar mass | 132.16 g/mol |
| Melting point | 140 °C (284 °F; 413 K) |
| soluble | |
| Solubility | soluble in ethanol |
| Acidity (pKa) | 1.94 |
Chiral rotation ([α]D) |
+11.5 (H2O, c = 6.5) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Role in urea cycle
L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen. Ornithine is recycled and, in a manner, is a catalyst. First, ammonia is converted into carbamoyl phosphate (H
2NC(O)OPO2−
3). Ornithine is converted into a urea derivative at the δ (terminal) nitrogen by carbamoyl phosphate synthetase. Another nitrogen is added from aspartate, producing the denitrogenated fumarate, and the resulting arginine (a guanidinium compound) is hydrolysed back to ornithine, producing urea. The nitrogens of urea come from the ammonia and aspartate, and the nitrogen in ornithine remains intact.
Ornithine is not an amino acid coded for by DNA, that is, not proteinogenic. However, in mammalian non-hepatic tissues, the main use of the urea cycle is in arginine biosynthesis, so, as an intermediate in metabolic processes, ornithine is quite important.[2]
Other reactions
Ornithine, via the action of ornithine decarboxylase (E.C. 4.1.1.17), is the starting point for the synthesis of polyamines such as putrescine.
In bacteria, such as E. coli, ornithine can be synthesized from L-glutamate.[3]
Research
Exercise fatigue
L-Ornithine supplementation attenuated fatigue in subjects in a placebo-controlled study using a cycle ergometer. The results suggested that L-ornithine has an antifatigue effect in increasing the efficiency of energy consumption and promoting the excretion of ammonia.[4][5]
Weightlifting supplement
Amino acid supplements, including L-ornithine, are frequently marketed to bodybuilders and weightlifters with claims for increasing levels of human growth hormone (HGH), muscle mass, and strength. A clinical study reported that L-ornithine at 2 g/d did not increase HGH.[6] A review on the topic concluded "The use of specific amino acids to stimulate GH release by athletes is not recommended."[7]
Cirrhosis
L-Ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, has been used in the treatment of cirrhosis.[8]
References
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-408. ISBN 0-8493-0462-8.
- Weber AL, Miller SL (1981). "Reasons for the occurrence of the twenty coded protein amino acids" (PDF). Journal of Molecular Evolution. 17 (5): 273–84. doi:10.1007/BF01795749. PMID 7277510.
- "Ornithine Biosynthesis". School of Biological and Chemical Sciences, Queen Mary, University of London. Archived from the original on 2012-04-14. Retrieved 2007-08-17. Cite journal requires
|journal=(help) - Sugino T, Shirai T, Kajimoto Y, Kajimoto O (November 2008). "L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism". Nutrition Research. 28 (11): 738–43. doi:10.1016/j.nutres.2008.08.008. PMID 19083482.
- Demura S, Yamada T, Yamaji S, Komatsu M, Morishita K (October 2010). "The effect of L-ornithine hydrochloride ingestion on performance during incremental exhaustive ergometer bicycle exercise and ammonia metabolism during and after exercise". European Journal of Clinical Nutrition. 64 (10): 1166–71. doi:10.1038/ejcn.2010.149. PMID 20717126.
- Fogelholm GM, Näveri HK, Kiilavuori KT, Härkönen MH (September 1993). "Low-dose amino acid supplementation: no effects on serum human growth hormone and insulin in male weightlifters". International Journal of Sport Nutrition. 3 (3): 290–7. doi:10.1123/ijsn.3.3.290. PMID 8220394.
- Chromiak JA, Antonio J (2002). "Use of amino acids as growth hormone-releasing agents by athletes". Nutrition. 18 (7–8): 657–61. doi:10.1016/s0899-9007(02)00807-9. PMID 12093449.
- Sikorska H, Cianciara J, Wiercińska-Drapało A (June 2010). "[Physiological functions of L-ornithine and L-aspartate in the body and the efficacy of administration of L-ornithine-L-aspartate in conditions of relative deficiency]". Polski Merkuriusz Lekarski. 28 (168): 490–5. PMID 20642112.