2-Aminoisobutyric acid
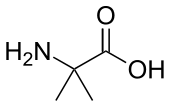
2-Aminoisobutyric acid, or α-aminoisobutyric acid (AIB) or α-methylalanine or 2-methylalanine, is the non-proteinogenic amino acid with the structural formula H2N-C(CH3)2-COOH. It is rare in nature only being found in meteorites[2], and some antibiotics of fungal origin, such as alamethicin and some lantibiotics.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-2-methylpropanoic acid | |
| Other names
α-Aminoisobutyric acid 2-Methylalanine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.495 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H9NO2 | |
| Molar mass | 103.12 g/mol |
| Appearance | white crystalline powder |
| Density | 1.09 g/mL |
| Boiling point | 204.4 °C (399.9 °F; 477.5 K) |
| soluble | |
| Acidity (pKa) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
In the laboratory, 2-aminoisobutyric acid may be prepared from acetone cyanohydrin, by reaction with ammonia followed by hydrolysis.[3] Industrial scale synthesis can be achieved by the selective hydroamination of methacrylic acid.
Biological activity
2-Aminoisobutyric acid is not one of the proteinogenic amino acids and is rather rare in nature (cf. non-proteinogenic amino acids). It is a strong helix inducer in peptides. Oligomers of AIB form 310 helices. 3-Aminoisobutyric acid, or BAIBA, is found as a normal metabolite of skeletal muscle in 2014. The plasma concentrations are increased in human by exercise. The production is likely a result of enhanced mitochondrial activity as the increase is also observed in the muscle of PGC-1a overexpression mice. BAIBA is proposed as protective factor against metabolic disorder since it can induce brown fat function.[4]
Ribosomal incorporation into peptides
Several reports have confirmed the compatibility of 2-aminoisobutyric acid with ribosomal elongation of peptide synthesis. Katoh et al. used flexizymes[5] and an engineered a tRNA body to enhance the affinity of aminoacylated AIB-tRNA species to elongation factor-P.[6] The result was an increased incorporation of AIB into peptides in a cell free translation system. Iqbal et al.. used an alternative approach of creating an editing deficient valine tRNA-ligase to synthesize aminoacylated AIB-tRNAVal. The aminoacylated tRNA was subsequently used in a cell-free translation system to yield AIB-containing peptides.[7]
References
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 5–88. ISBN 978-1498754286.
- "Immune System of Humans, Other Mammals Could Struggle to Fight Extraterrestrial Microorganisms". Science News. 23 July 2020. Retrieved 24 July 2020.
- Clarke, H. T.; Bean, H. J. (1931). "α-Aminoisobutyric acid". Organic Syntheses. 11: 4.; Collective Volume, 2, p. 29.
- Roberts, LD; Boström, P; O'Sullivan, JF; Schinzel, RT; Lewis, GD; Dejam, A; Lee, YK; Palma, MJ; Calhoun, S; Georgiadi, A; Chen, MH; Ramachandran, VS; Larson, MG; Bouchard, C; Rankinen, T; Souza, AL; Clish, CB; Wang, TJ; Estall, JL; Soukas, AA; Cowan, CA; Spiegelman, BM; Gerszten, RE (7 January 2014). "β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors". Cell Metabolism. 19 (1): 96–108. doi:10.1016/j.cmet.2013.12.003. PMC 4017355. PMID 24411942.
- Ohuchi, Masaki; Murakami, Hiroshi; Suga, Hiroaki (2007). "The flexizyme system: a highly flexible tRNA aminoacylation tool for the translation apparatus". Current Opinion in Chemical Biology. 11 (5): 537–542. doi:10.1016/j.cbpa.2007.08.011. PMID 17884697.
- Katoh, Takayuki; Iwane, Yoshihiko; Suga, Hiroaki (2017-12-15). "Logical engineering of D-arm and T-stem of tRNA that enhances d-amino acid incorporation". Nucleic Acids Research. 45 (22): 12601–12610. doi:10.1093/nar/gkx1129. ISSN 0305-1048. PMC 5728406. PMID 29155943.
- Iqbal, Emil S.; Dods, Kara K.; Hartman, Matthew C. T. (2018). "Ribosomal incorporation of backbone modified amino acids via an editing-deficient aminoacyl-tRNA synthetase". Organic & Biomolecular Chemistry. 16 (7): 1073–1078. doi:10.1039/c7ob02931d. ISSN 1477-0539. PMC 5993425. PMID 29367962.