Cerium oxalate
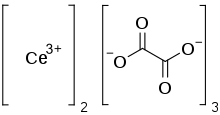
Cerium(III) oxalate (cerous oxalate) is the inorganic cerium salt of oxalic acid. It is a white crystalline solid with the chemical formula of Ce2(C2O4)3. It could be obtained by the reaction of oxalic acid with cerium(III) chloride.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Cerium(III) oxalate | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.875 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6Ce2O12 | |
| Molar mass | 544.286 g·mol−1 |
| Appearance | White crystals |
| Melting point | Decomposes |
| Slightly soluble | |
| Pharmacology | |
| A04AD02 (WHO) | |
| Hazards | |
| Main hazards | Corrosive, Irritant, Respiratory irritant, Toxic |
| Safety data sheet | External SDS |
| GHS pictograms |    |
| GHS Signal word | Danger[1] |
GHS hazard statements |
H301, H311, H314, H319, H331, H335, H370[1] |
| P260, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P308+313, P332+313, P403+233[1] | |
| NFPA 704 (fire diamond) | |
| Flash point | 188.8 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Cerium(III) oxalate is used as an antiemetic.[2][3] It has been identified as part of the invisible ink that was used by Stasi operatives during the Cold War.[4]
Toxicity
Cerium(III) oxalate irritates skin and mucous membranes, and is a strong irritant to eyes. If it gets into the eyes, there is a danger of severe eye injury.
Cerium salts increase the blood coagulation rate, and exposure to cerium salts can cause sensitivity to heat.
Oxalates are corrosive to tissue and are powerful irritants. They have a caustic effect on the linings of the digestive tracts and can cause kidney damage.
References
- "Cerium(III) Oxalate, Anhydrous". American Elements. Retrieved 2019-03-26.
- "KEGG DRUG: Cerium oxalate". KEGG DRUG Database. Retrieved 2019-03-26.
- Milne, G. W. A. (2017-11-01). Drugs: Synonyms and Properties: Synonyms and Properties. ISBN 9781351755092.
- "Cold War Invisible Ink Secrets Unlocked". ScienceDaily. 2006-11-08.
