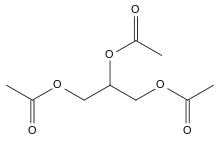
Triacetin
The triglyceride 1,2,3-triacetoxypropane is more generally known as triacetin, glycerin triacetate or 1,2,3-triacetylglycerol. It is the triester of glycerol and acetylating agents, such as acetic acid and acetic anhydride.[6] It is a colorless, viscous and odorless liquid at standard temperature and pressure (STP) with a high boiling point and a low melting point. It has a mild, sweet taste in concentrations lower than 500 ppm, but may appear bitter at higher concentrations.[7] It is one of the glycerine acetate compounds.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,3-Diacetyloxypropan-2-yl acetate | |
| Other names
Glycerol triacetate[2] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.775 |
| E number | E1518 (additional chemicals) |
| KEGG | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H14O6 | |
| Molar mass | 218.205 g·mol−1 |
| Appearance | Oily liquid |
| Density | 1.155 g/cm3[3] |
| Melting point | −78 °C (−108 °F; 195 K) at 760 mmHg[2] |
| Boiling point | 259 °C (498 °F; 532 K) at 760 mmHg[2] |
| 6.1 g/100 mL[2] | |
| Solubility | Miscible in EtOH Soluble in C6H6, (C2H5)2O, acetone[2] |
| Vapor pressure | 0.051 Pa (11.09 °C) 0.267 Pa (25.12 °C) 2.08 Pa (45.05 °C)[4] ln(P/Pa)=22.819-4493/T(K)-807000/T(K)² |
Refractive index (nD) |
1.4301 (20 °C)[2] 1.4294 (24.5 °C)[4] |
| Viscosity | 23 cP (20 °C)[3] |
| Thermochemistry | |
Heat capacity (C) |
389 J/mol·K[5] |
Std molar entropy (S |
458.3 J/mol·K[5] |
Std enthalpy of formation (ΔfH⦵298) |
−1330.8 kJ/mol[5] |
Std enthalpy of combustion (ΔcH⦵298) |
4211.6 kJ/mol[5] |
| Hazards | |
| S-phrases (outdated) | S24/25 |
| NFPA 704 (fire diamond) | |
| Flash point | 138 °C (280 °F; 411 K)[3] |
| 430 °C (806 °F; 703 K)[3] | |
| Explosive limits | 7.73%[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1100 mg/kg (mice, oral)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triacetin was first prepared in 1854 by the French chemist Marcellin Berthelot.[8]
Uses
It is an artificial chemical compound,[9] commonly used as a food additive, for instance as a solvent in flavourings, and for its humectant function, with E number E1518 and Australian approval code A1518. It is used as an excipient in pharmaceutical products, where it is used as a humectant, a plasticizer, and as a solvent.[10]
The plasticizing capabilities of triacetin have been utilized in the synthesis of a biodegradable phospholipid gel system for the dissemination of the cancer drug paclitaxel (PTX).[11] In the study, triacetin was combined with PTX, ethanol, a phospholipid and a medium chain triglyceride to form a gel-drug complex. This complex was then injected directly into the cancer cells of glioma-bearing mice. The gel slowly degraded and facilitated sustained release of PTX into the targeted glioma cells.
Additionally, preliminary research also suggests that triacetin can be used to directly treat glioblastoma[12]. The study found that triacetin is a viable mediator for acetate supplementation, a therapy that inhibits glioblastoma cell growth.
Triacetin can also be used as a fuel additive as an antiknock agent which can reduce engine knocking in gasoline, and to improve cold and viscosity properties of biodiesel.[13]
It has been considered as a possible source of food energy in artificial food regeneration systems on long space missions. It is believed to be safe to get over half of one's dietary energy from triacetin.[14]
Synthesis
The synthesis of triacetin from acetic anhydride and glycerol is simple and inexpensive.
- 3 (CH
3CO)
2O + 2 C
3H
5(OH)
3 → 2 C
3H
5(OCOCH
3)
3 + 3 H
2O
This synthesis has been conducted with catalytic sodium hydroxide and microwave irradiation to give a 99% yield of triacetin.[15] It has also been conducted with a cobalt(II) Salen complex catalyst supported by silicon dioxide and heated to 50 °C for 55 minutes to give a 99% yield of triacetin.[16]
Safety
The US Food and Drug Administration has approved it as Generally Recognized as Safe (GRAS) food additive and included it in the database according to the opinion from the Select Committee On GRAS Substances (SCOGS).
- "Triacetin and two types of acetooleins have been found to be without toxic effects in long-term feeding tests in rats at levels that were several orders of magnitude greater than those to which consumers are exposed. Three types of acetostearins have been found to be without toxic effects in long-term feeding tests in rats at levels up to 5 g per kg per day. This contrasts with an estimated human consumption of a fraction of a milligram per kg per day. It is recognized that at an even higher feeding level (10 g per kg per day) male rats developed testicular atrophy and female rats, uterine discoloration. However, such a level which would amount to 50 g or more for an infant and 600 g for an adult per day, is vastly higher than would be possible in the consumption of foods to which acetostearins are added for functional purposes."
Triacetin is included in the SCOGS database since 1975.[17]
Triacetin was not toxic to animals in studies of exposure through repeated inhalation over a relatively short period.[18]
References
- Merck Index (11th ed.). p. 9405.
- Lide DR, ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- "MSDS of Triacetin". fishersci.ca. Fisher Scientific. Retrieved 2014-06-20.
- Woodman AL, Adicoff A (1963). "Vapor Pressure of Tiracetin, Triethylene Glycol Dinitrate, and Metriol Trinitrate". Journal of Chemical & Engineering Data. 8 (2): 241–242. doi:10.1021/je60017a033.
- Triacetin in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov
- Kong P, Aroua MK, Daud WM, Lee HV, Cognet P, Pérès Y (2016). "Catalytic role of solid acid catalysts in glycerol acetylation for the production of bio-additives: a review". RSC Advances. 6 (73): 68885–68905. doi:10.1039/C6RA10686B.
- Arctander S (1969). Perfume and Flavor Chemicals (II K - Z). Elizabeth, N.J.: Published by the Author. p. 2971. ISBN 978-0931710377.
- Berthelot M (1854). "Sur les combinaisons de le glycérine avec les acides et sur la synthèse des principes immédiats des graisses des animaux" [On the compounds of glycerin with acids and on the synthesis of immediate principles of animal fats]. Annales de Chimie et de Physique. 3rd series (in French). 41: 216–319. ; see "Triacétine", pp. 282–283.
- "Triacetin". texas-chem.com. Chemical and Filtration Products of Texas. Retrieved 2014-06-20.
- "Triacetin". drugtopics.modernmedicine.com. Advanstar Communications, Inc. Archived from the original on 2012-02-19. Retrieved 2014-06-20.
- Chen T, Gong T, Zhao T, Liu X, Fu Y, Zhang Z, Gong T (August 2017). "Paclitaxel loaded phospholipid-based gel as a drug delivery system for local treatment of glioma". International Journal of Pharmaceutics. 528 (1–2): 127–132. doi:10.1016/j.ijpharm.2017.06.013. PMID 28596136.
- Long PM, Tighe SW, Driscoll HE, Fortner KA, Viapiano MS, Jaworski DM (August 2015). "Acetate supplementation as a means of inducing glioblastoma stem-like cell growth arrest". Journal of Cellular Physiology. 230 (8): 1929–43. doi:10.1002/jcp.24927. PMC 4414874. PMID 25573156.
- Gupta, Mayank; Kumar, Naveen (2012). "Scope and opportunities of using glycerol as an energy source" Renewable & Sustainable Energy Reviews". 16 (7): 4551–4556. doi:10.1016/j.rser.2012.04.001. Cite journal requires
|journal=(help) - Shapira J, Mandel AD, Quattrone PD, Bell NL (1968). "Current Research On Regenerative Systems" (PDF). casi.ntrs.nasa.gov. Tokyo: Committee On Space Research, Eleventh Annual Meeting. Retrieved 2014-06-20.
- Rajabi F, Saidi MR (2005). "A Cheap, Simple, and Versatile Method for Acetylation of Alcohols and Phenols and Selective Deprotection of Aromatic Acetates Under Solvent‐Free Condition". Synthetic Communications. 35 (3): 483–491. doi:10.1081/SCC-200048988. ISSN 0039-7911.
- Rajabi F (2009). "A heterogeneous cobalt(II) Salen complex as an efficient and reusable catalyst for acetylation of alcohols and phenols". Tetrahedron Letters. 50 (4): 395–397. doi:10.1016/j.tetlet.2008.11.024.
- "Glycerin and Glycerides". www.fda.gov. U.S. Food and Drug Administration. Retrieved 2014-06-20.
- Fiume MZ (2003). "Final report on the safety assessment of triacetin". International Journal of Toxicology. 22 Suppl 2 (3): 1–10. doi:10.1080/747398359. PMID 14555416.
