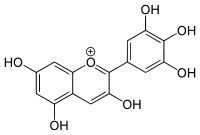
Delphinidin
Delphinidin (also delphinidine[1][2]) is an anthocyanidin, a primary plant pigment, and also an antioxidant.[3] Delphinidin gives blue hues to flowers in the genera Viola and Delphinium. It also gives the blue-red color of the grape that produces Cabernet Sauvignon, and can be found in cranberries and Concord grapes as well as pomegranates,[4] and bilberries.[5]
 | |
| Names | |
|---|---|
| IUPAC name
2-(3,4,5-Trihydroxyphenyl)chromenylium-3,5,7-triol | |
| Other names
3,3′,4′,5,5′,7-Hexahydroxyflavylium | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.671 |
| E number | E163b (colours) |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C15H11O7+ | |
| Molar mass | 303.24 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Delphinidin, like nearly all other anthocyanidins, is pH-sensitive, i.e. a natural pH indicator, and changes from red in acidic solution to blue in basic solution.
Glycosides
Several glycosides derived from delphinidin are known:
- Myrtillin (delphinidin-3-O-glucoside) and tulipanin (delphinidin-3-O-rutinoside) can be found in blackcurrant pomace.
- Violdelphin (delphinidin 3-rutinoside-7-O-(6-O-(4-(6-O-(4-hydroxybenzoyl)-β-D-glucosyl)oxybenzoyl)-β-D-glucoside) is responsible for the purplish-blue flower color of Aconitum chinense.[6]
- Nasunin (delphinidin-3-(p-coumaroylrutinoside)-5-glucoside) is responsible for the colour of the eggplant fruit's purple skin.[7]
gollark: Videos are generally kind of too slow-paced to actively hold my attention.
gollark: I watch longer (~30 minute) videos quite often, but mostly just put them on in the background when doing other stuff.
gollark: The UK doesn't really seem to have spoofing or nuisance calls anywhere near as much of the US, and I'm not sure why, given that it's technically possible in both.
gollark: The phone network really needs fixing to *not* allow spoofing.
gollark: <@617750798960558091> I briefly mistook that knife for a fish.
See also
- Prodelphinidin, a type of condensed tannins
References
- "Delphinidine".
- "Delphinidine".
- Afaq, F.; Syed, D. N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Zaid, M. A.; Mukhtar, H. (2007). "Delphinidin, an Anthocyanidin in Pigmented Fruits and Vegetables, Protects Human HaCaT Keratinocytes and Mouse Skin Against UVB-Mediated Oxidative Stress and Apoptosis". Journal of Investigative Dermatology. 127 (1): 222–232. doi:10.1038/sj.jid.5700510. PMID 16902416.
- Ribéreau-Gayon, J.; Ribéreau-Gayon, P. (1958). "The Anthocyans and Leucoanthocyans of Grapes and Wines". American Journal of Enology and Viticulture. 9: 1–9.
- Lätti AK, Riihinen KR, Kainulainen PS (2008). "Analysis of anthocyanin variation in wild populations of bilberry (Vaccinium myrtillus L.) in Finland". J Agric Food Chem. 56 (1): 190–6. doi:10.1021/jf072857m. PMID 18072741.
- CID 3083066 from PubChem
- Noda Y, Kneyuki T, Igarashi K, Mori A, Packer L (2000). "Antioxidant activity of nasunin, an anthocyanin in eggplant peels". Toxicology. 148: 119–23. doi:10.1016/s0300-483x(00)00202-x. PMID 10962130.CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.