Primulin (anthocyanin)
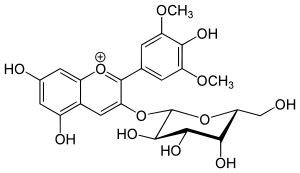
Primulin is an anthocyanin. It is the 3-galactoside of malvidin. It can be found in Primula sinensis.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3R,4S,5R,6R)-2-[5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)chromenylium-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol chloride | |
| Other names
Malvidin 3-galactoside Malvidin-3-galactoside chloride Malvidin-3-O-galactoside Malvidin-3-O-galactoside chloride 3-(Galactosyloxy)-5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-1-benzopyrylium chloride Primulin Yellow[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.045.490 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H25ClO12 C23H25O12+ | |
| Molar mass | 528.89 g/mol (chloride) 493.43 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The first crystalline form of this pigment was prepared by Rose Scott-Moncrieff in about 1930. This was the first crystalline anthrocyanine pigment ever identified. This was possible because of her insight into linking genetics with chemistry.[3]
References
- Primulin Yellow on chemicalregister.com
- J. B. Harborne; H. S. A. Sherratt (1961). "Plant Polyphenols: 3. Flavonoids in genotypes of Primula sinensis" (PDF). Biochem. J. 78: 298–306.
- Rose Scott-Moncrieff and the dawn of (Bio) Chemical Genetics, Cathie Martin, April 2016, Biochemical classics, Biochemist.org, Retrieved 5 July 2016
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.