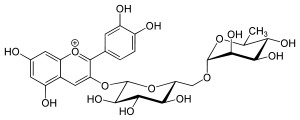
Antirrhinin
Antirrhinin is an anthocyanin. It is the 3-rutinoside of cyanidin.
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3R,4S,5S,6R)-2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromenylium-3-yl]oxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxane-3,4,5-triol chloride | |
| Other names
Keracyanin Prunicyanin Sambucin Cyaninoside Keraciannai Keracyanine Keracyaninum Cyanidin 3-rutinoside Cyanidin 3-O-rutinoside Keracyanin chloride cyanidin-3-rhamnoglucoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.038.646 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H31O15+, Cl− | |
| Molar mass | 630.97 g/mol (chloride) 595.52 g/mol (cation) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence
It can be found in Antirrhinum majus (common snapdragon).[1][2]
It can be found in blackcurrant,[3] açaí,[4] black raspberry,[5] litchi pericarp[6] and common fig.[7]
Metabolism
Cyanidin 3-O-rutinoside 5-O-glucosyltransferase uses UDP-glucose and cyanidin 3-O-rutinoside (antirrhinin) to produce UDP and cyanidin 3-O-rutinoside 5-O-beta-D-glucoside.
gollark: No.
gollark: That does not satisfy my values.
gollark: No.
gollark: If I unpause more, I MAY die.
gollark: No.
References
- Scott-Moncrieff, R (1930). "Natural anthocyanin pigments: The magenta flower pigment of Antirrhinum majus". Biochemical Journal. 24 (3): 753–766. doi:10.1042/bj0240753. PMC 1254517. PMID 16744416.
- Gilbert, R.I. (1971). "An unusual anthocyanin in Antirrhinum majus". Phytochemistry. 10 (11): 2848–2849. doi:10.1016/S0031-9422(00)97309-6.
- Slimestad, Rune; Solheim, Haavard (2002). "Anthocyanins from Black Currants (Ribes nigrumL.)". Journal of Agricultural and Food Chemistry. 50 (11): 3228–31. doi:10.1021/jf011581u. PMID 12009991.
- Gallori, S.; Bilia, A. R.; Bergonzi, M. C.; Barbosa, W. L. R.; Vincieri, F. F. (2004). "Polyphenolic Constituents of Fruit Pulp of Euterpe oleracea Mart. (Açai palm)". Chromatographia. 59 (11–12). doi:10.1365/s10337-004-0305-x.
- Tulio AZ, Reese RN, Wyzgoski FJ, Rinaldi PL, Fu R, Scheerens JC, Miller AR (2008). "Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry". Journal of Agricultural and Food Chemistry. 56 (6): 1880–8. doi:10.1021/jf072313k. PMID 18290621.
Five anthocyanins were present in black raspberries: cyanidin 3-sambubioside, cyanidin 3-glucoside, cyanidin 3-xylosylrutinoside, cyanidin 3-rutinoside, and pelargonidin 3-rutinoside. Their identities and structures, with particular emphasis on cyanidin 3-xylosylrutinoside, were confirmed by NMR spectroscopy. Two of these anthocyanins, cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside, predominated, comprising 24-40 and 49-58%, respectively, of the total anthocyanins in black raspberries. On the basis of both potency and concentration, cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside were found to be the significant contributors to the antioxidant systems of black raspberries.
- Sarni-Manchado, Pascale; Le Roux, Erwan; Le Guernevé, Christine; Lozano, Yves; Cheynier, Véronique (2000). "Phenolic Composition of Litchi Fruit Pericarp". Journal of Agricultural and Food Chemistry. 48 (12): 5995–6002. doi:10.1021/jf000815r. PMID 11312772.
- Solomon, A; Golubowicz, S; Yablowicz, Z; Grossman, S; Bergman, M; Gottlieb, HE; Altman, A; Kerem, Z; Flaishman, MA (2006). "Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.)". J Agric Food Chem. 54: 7717–23. doi:10.1021/jf060497h. PMID 17002444.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.