Fisetinidin
Fisetinidin is an anthocyanidin. It has been obtained from the heartwood of Acacia mearnsii,[1] from the bark of Rhizophora apiculata[2] and can also be synthesized.[3]
 | |
| Names | |
|---|---|
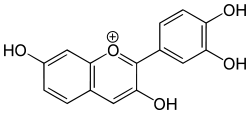
| IUPAC name
2-(3,4-dihydroxyphenyl)chromenylium-3,7-diol chloride | |
| Other names
Fisetinidin chloride 3,3',4',7-Tetrahydroxyflavylium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H11O5+ (Cl−) | |
| Molar mass | 306.69 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tannins
Fisetinidin can compose tannins.[1] The polymers are then called profisetinidin (Porter, 1992).[2]
gollark: It lacks most of the things which make Python particularly usable.
gollark: Have you *used* OCaml?
gollark: If you want to look at some projects written in Python, or some small code snippets, you can ask for that.
gollark: Like any general-purpose language, Python code can do basically anything which computers can do.
gollark: You see, communication (such as questions) involves multiple people.
References
- D. G. Roux; E. Paulus (February 1962). "Condensed tannins. 12. Polymeric leuco-fisetinidin tannins from the heartwood of Acacia mearnsii". Biochem. J. 82 (2): 320–324. doi:10.1042/bj0820320. PMC 1243455. PMID 14494576.
- Afidah A. Rahim; Emmanuel Rocca; Jean Steinmetz; M. Jain Kassim; M. Sani Ibrahim; Hasnah Osman (2008). "Antioxidant activities of mangrove Rhizophora apiculata bark extracts". Food Chemistry. 107 (1): 200–207. doi:10.1016/j.foodchem.2007.08.005.
- M. Gábor; E. EperJessy (10 December 1966). "Antibacterial Effect of Fisetin and Fisetinidin". Nature. 212 (1273): 1273. doi:10.1038/2121273a0. PMID 21090477.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.