Cytochrome P450 aromatic O-demethylase
Cytochrome P450 aromatic O-demethylase is a bacterial enzyme that catalyzes the demethylation of lignin and various lignols. The net reaction follows the following stoichiometry, illustrated with a generic methoxy arene:[1]
- ArOCH3 + O2 + 2 e− + 2 H+ → ArOH + CH2O + H2O
| Aromatic O-demethylase, cytochrome P450 subunit | |||||||
|---|---|---|---|---|---|---|---|
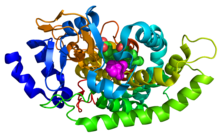 | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | gcoA | ||||||
| PDB | 5NCB | ||||||
| UniProt | P0DPQ7 | ||||||
| Other data | |||||||
| EC number | 1.14.14.- | ||||||
| |||||||
| Aromatic O-demethylase, reductase subunit | |||||||
|---|---|---|---|---|---|---|---|
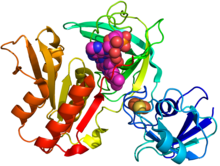 Crystal structure of gcoB (cartoon diagram) complexed with FAD (magenta spheres) and an iron–sulfur cluster (orange/yellow) based on PDB: 5OGX.[1] | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | gcoB | ||||||
| PDB | 5OGX | ||||||
| UniProt | P0DPQ8 | ||||||
| Other data | |||||||
| EC number | 1.6.2.- | ||||||
| |||||||
The enzyme is notable for its promiscuity, it effects the O-demethylation of a range of substrates, including lignin.
It is a heterodimeric protein derived from the products of two genes. The component proteins are a cytochrome P450 enzyme (encoded by the gcoA gene from the family CYP255A) and a three-domain reductase (encoded by the gcoB gene) complexed with three cofactors (2Fe-2S, FAD, and NADH).[1]
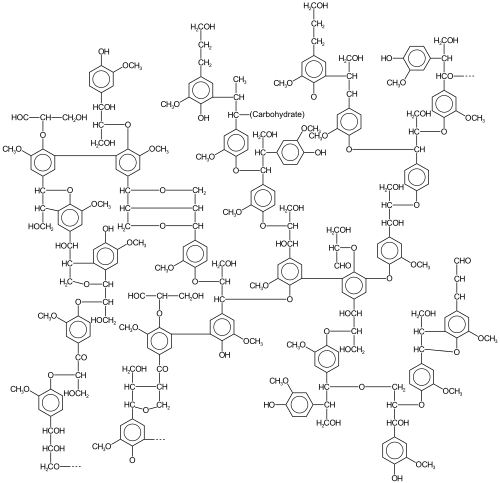
Mechanism
GcoA and GcoB form a dimer complex in solution. GcoA process the substrate while GcoB provides the electrons to support the mixed function oxidase. As with other P450's, monooxygenation of the substrate proceeds concomitantly with reduction of half an equivalent of O2 to water. An oxygen rebound mechanism can be assumed. GcoA positions the aromatic ring within the hydrophobic active site cavity where the heme is located.[2][3]
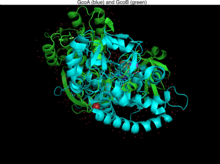
Structure
GcoA has a typical P450 structure: a thiolate-ligated heme next to a buried active site. GcoB is however unusual. Cytochrome P450s normally are complemented by either a cytochrome P450 reductase[4] or a ferredoxin and ferredoxin reductase; its electrons are carried by NAD+ or NADP+. GcoB however has a single polypeptide. This polypeptide has an N-terminal ferredoxin with both an NAD(P)+ and also an FAD binding region.
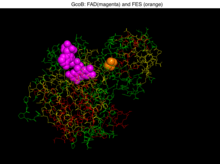
CcoA and GcoB are closely interlinked, acting as an heterodimer in solution. The surface of GcoB has an acidic patch that must interact with the matching basic region in GcoA. It is assumed that the part of GcoB interacting with GcoA is at the intersection between the FAD binding domain and ferredoxin domain. To achieve this GcoB would have to go through some structural change, which would represent a new class of P450 systems (family N).[5][6][7]
Potential applications
Cytochrome P450 aromatic O-demethylase assists in the partial O-demethylation of lignin. The resulting 1,2-diols are well suited for oxidative degradation via intra- and extra-diol dioxygenases. Thus O-demethylated lignins are potentially susceptible to partial depolymerization.[8] With fewer crosslinks, the modified ligand is potentially more useful than the precursor.,[9] ranging from fuels[10][11]
References
- Mallinson SJ, Machovina MM, Silveira RL, Garcia-Borràs M, Gallup N, Johnson CW, et al. (June 2018). "A promiscuous cytochrome P450 aromatic O-demethylase for lignin bioconversion". Nature Communications. 9 (1): 2487. Bibcode:2018NatCo...9.2487M. doi:10.1038/s41467-018-04878-2. PMC 6021390. PMID 29950589.
- Vaillancourt FH, Bolin JT, Eltis LD (2006). "The ins and outs of ring-cleaving dioxygenases". Critical Reviews in Biochemistry and Molecular Biology. 41 (4): 241–67. doi:10.1080/10409230600817422. PMID 16849108.
- Huang WC, Ellis J, Moody PC, Raven EL, Roberts GC (September 2013). "Redox-linked domain movements in the catalytic cycle of cytochrome p450 reductase". Structure. 21 (9): 1581–9. doi:10.1016/j.str.2013.06.022. PMC 3763376. PMID 23911089.
- Wang M, Roberts DL, Paschke R, Shea TM, Masters BS, Kim JJ (August 1997). "Three-dimensional structure of NADPH-cytochrome P450 reductase: prototype for FMN- and FAD-containing enzymes". Proceedings of the National Academy of Sciences of the United States of America. 94 (16): 8411–6. Bibcode:1997PNAS...94.8411W. doi:10.1073/pnas.94.16.8411. PMC 22938. PMID 9237990.
- Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL (March 1999). "Structure of a cytochrome P450-redox partner electron-transfer complex". Proceedings of the National Academy of Sciences of the United States of America. 96 (5): 1863–8. Bibcode:1999PNAS...96.1863S. doi:10.1073/pnas.96.5.1863. PMC 26702. PMID 10051560.
- Tripathi S, Li H, Poulos TL (June 2013). "Structural basis for effector control and redox partner recognition in cytochrome P450". Science. 340 (6137): 1227–30. Bibcode:2013Sci...340.1227T. doi:10.1126/science.1235797. PMID 23744947.
- Hasemann CA, Kurumbail RG, Boddupalli SS, Peterson JA, Deisenhofer J (January 1995). "Structure and function of cytochromes P450: a comparative analysis of three crystal structures". Structure. 3 (1): 41–62. doi:10.1016/s0969-2126(01)00134-4. PMID 7743131.
- Bugg TD, Rahmanpour R (December 2015). "Enzymatic conversion of lignin into renewable chemicals". Current Opinion in Chemical Biology. 29: 10–7. doi:10.1016/j.cbpa.2015.06.009. PMID 26121945.
- Beckham GT, Johnson CW, Karp EM, Salvachúa D, Vardon DR (December 2016). "Opportunities and challenges in biological lignin valorization". Current Opinion in Biotechnology. 42: 40–53. doi:10.1016/j.copbio.2016.02.030. PMID 26974563.
- Vardon DR, Franden MA, Johnson CW, Karp EM, Guarnieri MT, Linger JG, et al. (2015). "Adipic acid production from lignin". Energy & Environmental Science. 8 (2): 617–628. doi:10.1039/c4ee03230f. ISSN 1754-5692.
- Lin L, Cheng Y, Pu Y, Sun S, Li X, Jin M, et al. (2016). "Systems biology-guided biodesign of consolidated lignin conversion". Green Chemistry. 18 (20): 5536–5547. doi:10.1039/c6gc01131d. ISSN 1463-9262. OSTI 1326560.