Dimethyl acetylenedicarboxylate
Dimethyl acetylenedicarboxylate (DMAD) is an organic compound with the formula CH3O2CC2CO2CH3. It is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed as a dienophile in cycloaddition reactions, such as the Diels-Alder reaction. It is also a potent Michael acceptor.[1][2] This compound exists as a colorless liquid at room temperature. This compound was used in the preparation of nedocromil.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dimethyl but-2-ynedioate | |
| Other names
DMAD Acetylenedicarboxylic acid dimethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.999 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6O4 | |
| Molar mass | 142.11 g/mol |
| Appearance | Colorless liquid |
| Density | 1.1564 g/cm3 |
| Melting point | -18°C |
| Boiling point | 195 to 198 °C (383 to 388 °F; 468 to 471 K) (96–98° at 8 mm Hg) |
| Insoluble | |
| Solubility in other solvents | Soluble in most organic solvents |
Refractive index (nD) |
1.447 |
| Structure | |
| 0 D | |
| Hazards | |
| Main hazards | Toxic gas |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H302, H314 |
| P260, P264, P270, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P405, P501 | |
| Flash point | 187 °C (369 °F; 460 K) |
| Related compounds | |
Related compounds |
Methyl propiolate, Hexafluoro-2-butyne, Acetylene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
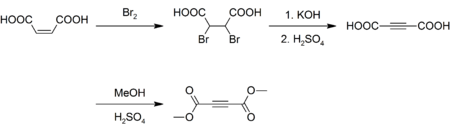
Preparation
Although inexpensively available, DMAD is prepared today as it was originally. Maleic acid is brominated and the resulting dibromosuccinic acid is dehydrohalogenated with potassium hydroxide yielding acetylenedicarboxylic acid.[3][4] The acid is then esterified with methanol and sulfuric acid as a catalyst:[5]
Safety
DMAD is a lachrymator and a vesicant.
References
- Stelmach, J. E.; Winkler, J. D. "Dimethyl Acetylenedicarboxylate"in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- Sahoo, Manoj (2007). "Dimethyl Acetylene Dicarboxylate". Synlett. 2007 (13): 2142–2143. doi:10.1055/s-2007-984894.
- Bandrowski, E. (1877). "Ueber Acetylendicarbonsäure". Berichte der Deutschen Chemischen Gesellschaft. 10: 838–842. doi:10.1002/cber.187701001231.
- Abbott, T. W.; Arnold, R. T.; Thompson, R. B. "Acetylenedicarboxylic acid". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 2, p. 10
- Huntress, E. H. Lesslie, T. E.; Bornstein, J. "Dimethyl Acetylenedicarboxylate". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 4, p. 329