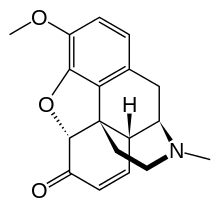
Codeinone
Codeinone is 1/3 as active as codeine as an analgesic but it is an important intermediate in the production of hydrocodone, a painkiller about 3/4 the potency of morphine; as well as of oxycodone.[1] The latter can also be synthesized from thebaine, however.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(5α)-7,8-didehydro-4,5-epoxy-3-methoxy-17-methylmorphinan-6-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.716 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H19NO3 | |
| Molar mass | 297.35 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemical structure
Codeinone can be described as the methylether of morphinone: 3-methyl-morphinone.
Codeinone can be also described as the ketone of codeine: codeine-6-one.
Apoptotic activity
Through renewed interest into possible anti-tumor activities of some of the opium alkaloids and derivatives, unrelated to their antinociceptive properties and habit-forming effects, the oxidation product of codeine has been found to induce cell death in three different human cancer cell lines in vitro.[3]
gollark: Try making the CC code also print the response it gets?
gollark: Hmm, I'd expect that to work.
gollark: That... seems sound, I think.
gollark: For your specific question could you post the code please?
gollark: As a general thing for Discord webhooks, I think you need to send a `Content-Type` header set to `application/json` and a reasonably unique `User-Agent` or it ignores you.
References
- Synthesis of Oxycodone from Codeine. Aug 2004 static snapshot of Rhodium site archive hosted by Erowid, May 2005
- Oxycodone / 14-hydroxydihydrocodeinone Synthesis; with alternative synthesis of 14-hydroxycodeinone intermediate. J. Med. Chem., 1974, 17, 1117
- Hitosugi N, Nagasaka H, Sakagami H, Matsumoto I, Kawase M (2003). Anticancer Res. 23(3B):2569-76. PMID 12894543
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.