Acalabrutinib
Acalabrutinib (trade name Calquence) is a medication used to treat a type of non-Hodgkin lymphoma known as mantle cell lymphoma.[2] Specifically it is for people who had previously been treated with another therapy.[3] As of 2019, it was unclear whether it improved outcomes.[2]
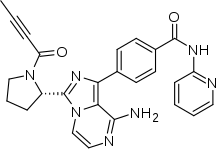 | |
| Clinical data | |
|---|---|
| Trade names | Calquence |
| Other names | ACP-196 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.247.121 |
| Chemical and physical data | |
| Formula | C26H23N7O2 |
| Molar mass | 465.517 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Common side effects include headaches, feeling tired, low red blood cells, low platelets, and low white blood cells.[2] It is a second generation Bruton's tyrosine kinase inhibitor.[4][5]
Acalabrutinib was approved for medical use in the United States in 2017.[2] The wholesale cost as of 2018 in the United States is US$14,064 per month.[6]
Medical uses
It is used to treat a type of non-Hodgkin lymphoma known as mantle cell lymphoma.[2] It is unclear if it results in improved outcomes as of 2019.[2]
Side effects
The most common adverse events were headache, diarrhea and weight gain.[5] Despite the appearance of a greater occurrence of transient headaches, data suggest a preferred advantage of acalabrutinib over ibrutinib due to expected reduced adverse events of skin rash, severe diarrhea, and bleeding risk.[5]
Society and culture
Regulatory
As of February 2016, acalabrutinib had received orphan drug designation in the United States for mantle cell lymphoma and chronic lymphocytic leukemia (CLL),[8] [9] and was similarly designated as an orphan medicinal product by the European Medicines Agency (EMA) Committee for Orphan Medicinal Products (COMP) for treatment of three indications - CLL/ small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), and lymphoplasmacytic lymphoma (Waldenström's macroglobulinaemia, WM).[10][11][12][13] Approval would result in a 10-year period of market exclusivity for the stated indications within Europe.[14]
Economics
It was developed by Acerta Pharma.[15] After promising results for CLL in initial clinical trials,[4] Astra Zeneca purchased a 55% stake in Acerta Pharma for $4 billion in December 2015, with an option to acquire the remaining 45% stake for an additional $3 billion, conditional on approval in both the US and Europe and the establishment of commercial opportunity.[16]
Research
Relative to ibrutinib, acalabrutinib demonstrated higher selectivity and inhibition of the targeted activity of BTK, while having a much greater IC50 or otherwise virtually no inhibition on the kinase activities of ITK, EGFR, ERBB2, ERBB4, JAK3, BLK, FGR, FYN, HCK, LCK, LYN, SRC, and YES1.[5] In addition, in platelets treated with ibrutinib, thrombus formation was clearly inhibited while no impact to thrombus formation was identified relative to controls for those treated with acalabrutinib.[5] These findings strongly suggest an improved safety profile of acalabrutinib with minimized adverse effects relative to ibrutinib.[5] In pre-clinical studies, it was shown to be more potent and selective than ibrutinib, the first-in-class BTK inhibitor.[4][5][17]
The interim results of the still on-going first human phase 1/2 clinical trial (NCT02029443) with 61 patients for the treatment of relapsed chronic lymphocytic leukemia (CLL) are encouraging, with a 95% overall response rate demonstrating potential to become a best-in-class treatment for CLL.[4] Notably, a 100% response rate was achieved for those people which were positive for the 17p13.1 gene deletion - a subgroup that typically results in a poor response to therapy and expected outcomes.[5]
References
- "Acalabrutinib (Calquence) Use During Pregnancy". Drugs.com. 23 October 2019. Retrieved 28 March 2020.
- "Acalabrutinib Monograph for Professionals". Drugs.com. Retrieved 16 March 2019.
- "FDA approves new treatment for adults with mantle cell lymphoma". U.S. Food and Drug Administration (FDA) (Press release). 31 October 2017. Retrieved 28 March 2020.
- Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. (January 2016). "Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia". The New England Journal of Medicine. 374 (4): 323–32. doi:10.1056/NEJMoa1509981. PMC 4862586. PMID 26641137.
- Wu J, Zhang M, Liu D (March 2016). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9: 21. doi:10.1186/s13045-016-0250-9. PMC 4784459. PMID 26957112.
- "In Brief: Acalabrutinib (Calquence) for Mantle Cell Lymphoma (online only)". The Medical Letter. Retrieved 16 March 2019.
- "WHO Drug Information - recommended INN" (PDF). WHO Drug Information. World Health Oorganisation. Retrieved 24 December 2015.
- "Acalabrutinib Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). Retrieved 15 April 2020.
- "Acalabrutinib Orphan Drug Designation and Approval". U.S. Food and Drug Administration (FDA). Retrieved 15 April 2020.
- "EU/3/16/1624". European Medicines Agency (EMA). 2 May 2016. Retrieved 15 April 2020.
- "EU/3/16/1625". European Medicines Agency (EMA). 4 May 2016. Retrieved 15 April 2020.
- "EU/3/16/1626". European Medicines Agency (EMA). 4 May 2016. Retrieved 15 April 2020.
- "azn201602256k.htm". www.sec.gov. Retrieved 2016-11-21.
- House DW (2016-02-25). "AstraZeneca and Acerta Pharma's acalabrutinib tagged an Orphan Drug in Europe for three indications". Seeking Alpha. Retrieved 2016-11-21.
- "AstraZeneca to buy Acerta for blood cancer drug". www.rsc.org. Chemistry World - Royal Society of Chemistry. Retrieved 24 December 2015.
- Walker I, Roland D (2015-12-17). "AstraZeneca to Buy Stake in Acerta Pharma". Wall Street Journal. ISSN 0099-9660. Retrieved 2016-11-19.
- Wu J, Zhang M, Liu D (March 2016). "Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor". Journal of Hematology & Oncology. 9 (1): 21. doi:10.1186/s13045-016-0250-9. PMC 4784459. PMID 26957112.
External links
- "Acalabrutinib". Drug Information Portal. U.S. National Library of Medicine.
- "Acalabrutinib". National Cancer Institute.