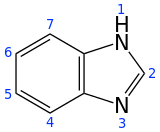
Benzimidazole
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and imidazole. It is a colorless solid.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1H-1,3-Benzimidazole | |||
| Other names
1H-Benzo[d]imidazole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 109682 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.075 | ||
| EC Number |
| ||
| 3106 | |||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H6N2 | |||
| Molar mass | 118.139 g·mol−1 | ||
| Melting point | 170 to 172 °C (338 to 342 °F; 443 to 445 K) | ||
| Acidity (pKa) | 12.8 (for benzimidazole) and 5.6 (for the conjugate acid) [1] | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms |  | ||
| GHS Signal word | Warning | ||
GHS hazard statements |
H302, H315, H319, H335 | ||
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation
Benzimidazole is produced by condensation of o-phenylenediamine with formic acid,[2] or the equivalent trimethyl orthoformate:
- C6H4(NH2)2 + HC(OCH3)3 → C6H4N(NH)CH + 3 CH3OH
2-substituted derivatives are obtained when the condensation is conducted with aldehydes in place of formic acid, followed by oxidation. This method afford 2-substituted benzimidazoles.[3]
Reactions
Benzimidazole is a base:
- C6H4N(NH)CH + H+ → [C6H4(NH)2CH]+
It can also be deprotonated with stronger bases:
- C6H4N(NH)CH + LiH → Li [C6H4N2CH] + H2
The imine can be alkylated and also serves as a ligand in coordination chemistry. The most prominent benzimidazole complex features N-ribosyl-dimethylbenzimidazole as found in vitamin B12.[4]
N,N'-Dialkylbenzimidazolium salts are precursors to certain N-heterocyclic carbenes.[5][6]
Applications
Benzimidazoles are often bioactive. Many anthelmintic drugs (albendazole, mebendazole, triclabendazole etc.) belong to the benzimidazole class of compounds. Benzimidazole fungicides are commercialized. They act by binding to the fungal microtubules and stopping hyphal growth. It also binds to the spindle microtubules and blocks nuclear division.
The proton-pump inhibitors (antacids) omeprazole, lansoprazole, pantoprazole, rabeprazole, and tenatoprazole all contain a benzimidazole group. Other pharmaceutical drugs which contain a benzimidazole group include etonitazene, galeterone, mavatrep, and dovitinib. Benzimidazole derivatives are among the top frequently used ring systems for small molecule drugs listed by the US FDA. [7]
In printed circuit board manufacturing, benzimidazole can be used as an organic solderability preservative.
Several dyes are derived from benzimidazoles.[8]
See also
- Benzimidazoline
- Polybenzimidazole, a high performance fiber
References
- Walba, H. & Isensee, R. W. Acidity constants of some arylimidazoles and their cations. J. Org. Chem. 26, 2789-2791 (1961).
- E. C. Wagner and W. H. Millett (1943). "Benzimidazole". Organic Syntheses.; Collective Volume, 2, p. 65.
- Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405
- H. A. Barker; R. D. Smyth; H. Weissbach; J. I. Toohey; J. N. Ladd & B. E. Volcani (February 1, 1960). "Isolation and Properties of Crystalline Cobamide Coenzymes Containing Benzimidazole or 5,6-Dimethylbenzimidazole". Journal of Biological Chemistry. 235 (2): 480–488. PMID 13796809.
- R. Jackstell; A. Frisch; M. Beller; D. Rottger; M. Malaun; B. Bildstein (2002). "Efficient telomerization of 1,3-butadiene with alcohols in the presence of in situ generated palladium(0)carbene complexes". Journal of Molecular Catalysis A: Chemical. 185 (1–2): 105–112. doi:10.1016/S1381-1169(02)00068-7.
- H. V. Huynh; J. H. H. Ho; T. C. Neo; L. L. Koh (2005). "Solvent-controlled selective synthesis of a trans-configured benzimidazoline-2-ylidene palladium(II) complex and investigations of its Heck-type catalytic activity". Journal of Organometallic Chemistry. 690 (16): 3854–3860. doi:10.1016/j.jorganchem.2005.04.053.
- Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J Med Chem 2014, 57, 5845.>
- Horst Berneth "Methine Dyes and Pigments" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a16_487.pub2
Further reading
- Grimmett, M. R. (1997). Imidazole and benzimidazole synthesis. Boston: Academic Press. ISBN 0-12-303190-7.