Mebendazole
Mebendazole (MBZ) is a medication used to treat a number of parasitic worm infestations.[3] This includes ascariasis, pinworm disease, hookworm infections, guinea worm infections, hydatid disease, and giardia, among others.[3] It is taken by mouth.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Vermox[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682315 |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 2–10% |
| Protein binding | 95% |
| Metabolism | Extensive liver |
| Elimination half-life | 3–6 hours |
| Excretion | Faeces, urine (5–10%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.017 |
| Chemical and physical data | |
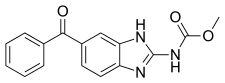
| Formula | C16H13N3O3 |
| Molar mass | 295.298 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 288.5 °C (551.3 °F) |
| |
| |
| (verify) | |
Mebendazole is usually well tolerated.[3] Common side effects include headache, vomiting, and ringing in the ears.[3] If used at large doses it may cause bone marrow suppression.[3] It is unclear if it is safe in pregnancy.[3] Mebendazole is a broad-spectrum antihelminthic agent of the benzimidazole type.[3]
Mebendazole came into use in 1971, after it was developed by Janssen Pharmaceutica in Belgium.[4] It is on the World Health Organization's List of Essential Medicines.[5] Mebendazole is available as a generic medication.[6]
Medical use
Mebendazole is a highly effective, broad-spectrum antihelmintic indicated for the treatment of nematode infestations, including roundworm, hookworm, whipworm, threadworm, pinworm, and the intestinal form of trichinosis prior to its spread into the tissues beyond the digestive tract. Other drugs are used to treat worm infections outside the digestive tract, as mebendazole is poorly absorbed into the bloodstream.[7] Mebendazole is used alone in those with mild to moderate infestations. It kills parasites relatively slowly, and in those with very heavy infestations, it can cause some parasites to migrate out of the digestive system, leading to appendicitis, bile duct problems, or intestinal perforation. To avoid this, heavily infested patients may be treated with piperazine, either before or instead of mebendazole. Piperazine paralyses the parasites, causing them to pass in the feces.[8] It is also used rarely in the treatment of cystic echinococcosis, also known as hydatid disease. Evidence for effectiveness for this disease, however, is poor.[9]
Mebendazole and other benzimidazole antithelmetics are active against both larval and adult stages of nematodes, and in the cases of roundworm and whipworm, kill the eggs, as well. Paralysis and death of the parasites occurs slowly, and elimination in the feces may require several days.[7]
Special populations
Mebendazole is pregnancy category C, which means it has been shown to cause ill effects in pregnancy in animal models, and no adequate studies of its effects in human pregnancy have been conducted. Whether it can be passed by breastfeeding is unknown.[10]
Adverse effects
Mebendazole sometimes causes diarrhea, abdominal pain, and elevated liver enzymes. In rare cases, it has been associated with a dangerously low white blood cell count, low platelet count, and hair loss,[10][11] with a risk of agranulocytosis in rare cases
Drug interactions
Carbamazepine and phenytoin lower serum levels of mebendazole. Cimetidine does not appreciably raise serum mebendazole (in contrast to the similar drug albendazole), consistent with its poor systemic absorption.[12][13]
Stevens–Johnson syndrome and the more severe toxic epidermal necrolysis can occur when mebendazole is combined with high doses of metronidazole.[14]
Mechanism
Mebendazole works by selectively inhibiting the synthesis of microtubules via binding to colchicine binding site of β-tubulin, thereby blocking polymerisation of tubulin dimers in intestinal cells of parasites.[15] Disruption of cytoplasmic microtubules leads to blocking the uptake of glucose and other nutrients, resulting in the gradual immobilization and eventual death of the helminths.[7]
Poor absorption in digestive tract makes mebendazole an efficient drug for treating intestinal parasitic infections with limited adverse effects. However mebendazole has impact on mammalian cells mostly by inhibiting polymeration of tubulin dimers, thereby disrupting essential microtubule structures such as mitotic spindle.[16] Disassembly of mitotic spindle then leads to apoptosis mediated via dephosphorylation of Bcl-2 which allows pro-apoptotic protein Bax to dimerize and innitiate programmed cell death.[17]
Society and culture
Availability
Mebendazole is available as a generic medication.[6] Mebendazole is distributed in international markets by Johnson and Johnson and a number of generic manufacturers.[18]
Cost
In the United States a single dose was about US$18.00 in 2015.[3] In 2016 the price increased to US$440.00 per dose in the U.S. as Amedra Pharmaceuticals acquired the rights from Teva in 2013.[19]
In 2010, Amedra also bought the U.S. marketing rights to the only other interchangeable anti-parasitic medication, albendazole, from GSK. The result of these acquisitions created a monopoly on these medications and the price increased dramatically.[20] In 2015 Amedra Pharmaceuticals became a subsidiary of Amneal Pharmaceuticals.
In Australia 6 x 100mg costs approximately US$9 while in the UK this would cost around US$19.[19]
Research
Several studies show mebendazole exhibits potent antitumor properties. MBZ significantly inhibited cancer cell growth, migration, and metastatic formation of adrenocortical carcinoma, both in vitro and in vivo.[21] Treatment of lung cancer cell lines with MBZ caused mitotic arrest, followed by apoptotic cell death with the feature of caspase activation and cytochrome c release.[22] MBZ induced a dose- and time-dependent apoptotic response in human lung cancer cell lines,[23] and apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells.[24] The anti-cancer effect of mebendazole comes from preclinical studies and case reports.[25]
References
- Ebadi M (2008). Desk reference of clinical pharmacology (2nd ed.). Boca Raton: CRC Press. p. 403. ISBN 9781420047448. Archived from the original on 2017-09-08.
- "MEBENDAZOLE". Archived from the original on 2016-10-23. Retrieved 2016-04-29.
- "Mebendazole". The American Society of Health-System Pharmacists. Archived from the original on 2015-09-07. Retrieved Aug 18, 2015.
- Mehlhorn, Heinz (2001). Encyclopedic reference of parasitology. 107 tables (2 ed.). Berlin [u.a.]: Springer. p. 259. ISBN 9783540668299. Archived from the original on 2017-09-08.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- Hamilton, Richard J. (2012). Tarascon pocket pharmacopoeia (13 ed.). Burlington, Mass.: Jones & Bartlett Learning. p. 33. ISBN 9781449624286. Archived from the original on 2017-09-08.
- Petri WA in Brunton LL, Chabner BA, Knollmann BC, Ed. Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12th ed., Chapter 42. McGraw-Hill, 2011 New York.
- Martin AR in Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical Chemistry, 8th edition, Doerge RF, ed. J.B. Lippincott, 1982, Chapter 4
- "Mebendazole". drugs.com. Archived from the original on 22 February 2015. Retrieved 25 January 2015.
- Finberg R, Fingeroth J in Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo, Ed. Harrison's Principles of Internal Medicine, 18th ed., McGraw-Hill, 2012, Chapter 217.
- Andersohn F, Konzen C, Garbe E (May 2007). "Systematic review: agranulocytosis induced by nonchemotherapy drugs". Annals of Internal Medicine. 146 (9): 657–65. doi:10.7326/0003-4819-146-9-200705010-00009. PMID 17470834.
- "Drug Interactions". Medicine chest. Archived from the original on 2007-02-06. Retrieved 2008-05-06.
- Luder PJ, Siffert B, Witassek F, Meister F, Bircher J (1986). "Treatment of hydatid disease with high oral doses of mebendazole. Long-term follow-up of plasma mebendazole levels and drug interactions". European Journal of Clinical Pharmacology. 31 (4): 443–8. doi:10.1007/bf00613522. PMID 3816925.
- Chen KT, Twu SJ, Chang HJ, Lin RS (March 2003). "Outbreak of Stevens-Johnson syndrome/toxic epidermal necrolysis associated with mebendazole and metronidazole use among Filipino laborers in Taiwan". American Journal of Public Health. 93 (3): 489–92. doi:10.2105/ajph.93.3.489. PMC 1447769. PMID 12604501.
- Lacey E (April 1990). "Mode of action of benzimidazoles". Parasitology Today. 6 (4): 112–5. doi:10.1016/0169-4758(90)90227-U. PMID 15463312.
- De Witt M, Gamble A, Hanson D, Markowitz D, Powell C, Al Dimassi S, et al. (April 2017). "Repurposing Mebendazole as a Replacement for Vincristine for the Treatment of Brain Tumors". Molecular Medicine. 23: 50–56. doi:10.2119/molmed.2017.00011. PMC 5403762. PMID 28386621.
- Blagosklonny MV, Giannakakou P, el-Deiry WS, Kingston DG, Higgs PI, Neckers L, Fojo T (January 1997). "Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death". Cancer Research. 57 (1): 130–5. PMID 8988053.
- Global Pharmaceutical Pricing and Reimbursement Database, zenRx Research, archived from the original on 30 June 2015, retrieved 2014-06-12
- Crow, David (18 December 2016). "US drugmaker charges 200 times UK price for common worm pill". www.ft.com. Archived from the original on 8 January 2017. Retrieved 8 January 2017.
- Uhl K, Peters JR, Flanagan K (February 2015). "High-cost generic drugs--implications for patients and policymakers". The New England Journal of Medicine. 372 (7): 685–6. doi:10.1056/NEJMc1415471. PMID 25671270.
- Martarelli D, Pompei P, Baldi C, Mazzoni G (April 2008). "Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice". Cancer Chemotherapy and Pharmacology. 61 (5): 809–17. doi:10.1007/s00280-007-0538-0. PMID 17581752.
- Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth JA, Mukhopadhyay T (November 2002). "The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells". Molecular Cancer Therapeutics. 1 (13): 1201–9. PMID 12479701.
- Mukhopadhyay T, Sasaki J, Ramesh R, Roth JA (September 2002). "Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo". Clinical Cancer Research. 8 (9): 2963–9. PMID 12231542.
- Doudican N, Rodriguez A, Osman I, Orlow SJ (August 2008). "Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells". Molecular Cancer Research. 6 (8): 1308–15. doi:10.1158/1541-7786.MCR-07-2159. PMID 18667591.
- Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP (2014). "Repurposing Drugs in Oncology (ReDO)-mebendazole as an anti-cancer agent". Ecancermedicalscience. 8: 443. doi:10.3332/ecancer.2014.443. PMC 4096024. PMID 25075217.
External links
- "Mebendazole". Drug Information Portal. U.S. National Library of Medicine.