Ferrate(VI)
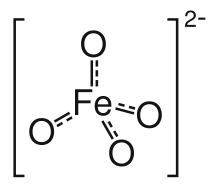
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH.
 | |
 Solutions of ferrate (left) and permanganate (right) | |
| Names | |
|---|---|
| IUPAC name
Ferrate(VI) | |
| Systematic IUPAC name
Tetraoxoironbis(olate) | |
| Other names
[FeO4]2- | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| [FeO4]2- | |
| Molar mass | 119.843 g mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nomenclature
The term ferrate is normally used to mean ferrate(VI), although it can refer to other iron-containing anions, many of which are more commonly encountered than salts of [FeO4]2−. These include the highly reduced species disodium tetracarbonylferrate Na
2[Fe(CO)
4], K
2[Fe(CO)
4] and salts of the iron(III) complex tetrachloroferrate [FeCl4]− in 1-Butyl-3-methylimidazolium tetrachloroferrate. Although rarely studied, ferrate(V) [FeO4]3− and ferrate(IV) [FeO4]4− oxyanions of iron also exist. These too are called ferrates.[1]
Synthesis
Ferrate(VI) salts are formed by oxidizing iron in an aqueous medium with strong oxidizing agents under alkaline conditions, or in the solid state by heating a mixture of iron filings and powdered potassium nitrate.[2]
For example, ferrates are produced by heating iron(III) hydroxide with sodium hypochlorite in alkaline solution:[3]
The anion is typically precipitated as the barium(II) salt, forming barium ferrate.[3]
Properties
The ferrate(VI) anion is unstable at neutral[2] or acidic pH values, decomposing to iron(III):[3]
The reduction goes through intermediate species in which iron has oxidation states +5 and +4.[4] These anions are even more reactive than ferrate(VI).[5] In alkaline conditions ferrates are more stable, lasting for about 8 to 9 hours at pH 8 or 9.[5]
Aqueous solutions of ferrates are pink when dilute, and deep red or purple at higher concentrations.[4][6] The ferrate ion is a stronger oxidizing agent than permanganate,[7] and will oxidize chromium(III) to dichromate,[8] and ammonia to molecular nitrogen.[9]
Ferrates are excellent disinfectants, and are capable of removing and destroying viruses.[10]
The ferrate(VI) ion has two unpaired electrons, and is thus paramagnetic. It has a tetrahedral molecular geometry.[4]
Fe(VI) is a strong oxidizing agent over the entire pH range, with a reduction potential (Fe(VI)/Fe(III) couple) varying from +2.2 V to +0.7 V versus NHE in acidic and basic media respectively. Sodium ferrate (Na
2FeO
4) is a useful reagent with good selectivity and is stable in aqueous solution of high pH, remaining soluble in an aqueous solution saturated with sodium hydroxide. Iron usually exists in the +2 and +3 oxidation states; however, in a strong oxidizing environment, higher oxidation states of iron such as +4, +5 and +6 can also be obtained.
Ferrate is one of the most powerful, yet environmentally friendly, water treatment chemicals known. The byproduct of ferrate oxidation is the relatively benign iron(III), or ferric oxide. Fe(III) occurs naturally in the human body and plant and animal forms. Ferrate is a tetrahedral ion isostructural with the chromate and permanganate ions.
See also
References
- Graham Hill; John Holman (2000). Chemistry in context (5th ed.). Nelson Thornes. p. 202. ISBN 0-17-448276-0.
- R. K. Sharma (2007). Text Book Of Coordination Chemistry. Discovery Publishing House. pp. 124–125. ISBN 81-8356-223-X.
- Gary Wulfsberg (1991). Principles of descriptive inorganic chemistry. University Science Books. pp. 142–143. ISBN 0-935702-66-0.
- Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. pp. 1457–1458. ISBN 0-12-352651-5.
- Gary M. Brittenham (1994). Raymond J. Bergeron (ed.). The Development of Iron Chelators for Clinical Use. CRC Press. pp. 37–38. ISBN 0-8493-8679-9.
- John Daintith, ed. (2004). Oxford dictionary of chemistry (5th ed.). Oxford University Press. p. 235. ISBN 0-19-860918-3.
- Kenneth Malcolm Mackay; Rosemary Ann Mackay; W. Henderson (2002). Introduction to modern inorganic chemistry (6th ed.). CRC Press. pp. 334–335. ISBN 0-7487-6420-8.
- Amit Arora (2005). Text Book Of Inorganic Chemistry. Discovery Publishing House. pp. 691–692. ISBN 81-8356-013-X.
- Karlis Svanks (June 1976). "Oxidation of Ammonia in Water by Ferrates(VI) and (IV)" (PDF). Water Resources Center, Ohio State University. p. 3. Retrieved 2010-05-04.
- Stanley E. Manahan (2005). Environmental chemistry (8th ed.). CRC Press. p. 234. ISBN 1-56670-633-5.