Plant defense against herbivory
Plant defense against herbivory or host-plant resistance (HPR) describes a range of adaptations evolved by plants which improve their survival and reproduction by reducing the impact of herbivores. Plants can sense being touched,[1] and they can use several strategies to defend against damage caused by herbivores. Many plants produce secondary metabolites, known as allelochemicals, that influence the behavior, growth, or survival of herbivores. These chemical defenses can act as repellents or toxins to herbivores, or reduce plant digestibility.

Other defensive strategies used by plants include escaping or avoiding herbivores in any time and/or any place, for example by growing in a location where plants are not easily found or accessed by herbivores, or by changing seasonal growth patterns. Another approach diverts herbivores toward eating non-essential parts, or enhances the ability of a plant to recover from the damage caused by herbivory. Some plants encourage the presence of natural enemies of herbivores, which in turn protect the plant. Each type of defense can be either constitutive (always present in the plant), or induced (produced in reaction to damage or stress caused by herbivores).
Historically, insects have been the most significant herbivores, and the evolution of land plants is closely associated with the evolution of insects. While most plant defenses are directed against insects, other defenses have evolved that are aimed at vertebrate herbivores, such as birds and mammals. The study of plant defenses against herbivory is important, not only from an evolutionary view point, but also in the direct impact that these defenses have on agriculture, including human and livestock food sources; as beneficial 'biological control agents' in biological pest control programs; as well as in the search for plants of medical importance.
Evolution of defensive traits
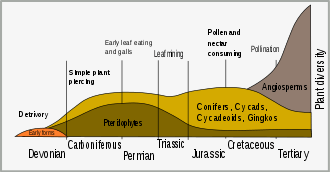
The earliest land plants evolved from aquatic plants around 450 million years ago (Ma) in the Ordovician period. Many plants have adapted to iodine-deficient terrestrial environment by removing iodine from their metabolism, in fact iodine is essential only for animal cells.[2] An important antiparasitic action is caused by the block of the transport of iodide of animal cells inhibiting sodium-iodide symporter (NIS). Many plant pesticides are glycosides (as the cardiac digitoxin) and cyanogenic glycosides which liberate cyanide, which, blocking cytochrome c oxidase and NIS, is poisonous only for a large part of parasites and herbivores and not for the plant cells in which it seems useful in seed dormancy phase. Iodide is not a pesticide, but is oxidized, by vegetable peroxidase, to iodine, which is a strong oxidant, able to kill bacteria, fungi and protozoa.[3]
The Cretaceous period saw the appearance of more plant defense mechanisms. The diversification of flowering plants (angiosperms) at that time is associated with the sudden burst of speciation in insects.[4] This diversification of insects represented a major selective force in plant evolution, and led to selection of plants that had defensive adaptations. Early insect herbivores were mandibulate and bit or chewed vegetation; but the evolution of vascular plants lead to the co-evolution of other forms of herbivory, such as sap-sucking, leaf mining, gall forming and nectar-feeding.[5]
The relative abundance of different species of plants in ecological communities including forests and grasslands may be determined in part by the level of defensive compounds in the different species.[6] Since the cost of replacement of damaged leaves is higher in conditions where resources are scarce, it may also be that plants growing in areas where water and nutrients are scarce may invest more resources into anti-herbivore defenses.
Records of herbivores

Our understanding of herbivory in geological time comes from three sources: fossilized plants, which may preserve evidence of defense (such as spines), or herbivory-related damage; the observation of plant debris in fossilised animal feces; and the construction of herbivore mouthparts.[7]
Long thought to be a Mesozoic phenomenon, evidence for herbivory is found almost as soon as fossils which could show it. As previously discussed, the first land plants emerged around 450 million years ago; however, herbivory, and therefore the need for plant defenses, has undoubtedly been around for longer. Herbivory first evolved due to marine organisms within ancient lakes and oceans.[8] Within under 20 million years of the first fossils of sporangia and stems towards the close of the Silurian, around 420 million years ago, there is evidence that they were being consumed.[9] Animals fed on the spores of early Devonian plants, and the Rhynie chert also provides evidence that organisms fed on plants using a "pierce and suck" technique.[7] Many plants of this time are preserved with spine-like enations, which may have performed a defensive role before being co-opted to develop into leaves.
During the ensuing 75 million years, plants evolved a range of more complex organs – from roots to seeds. There was a gap of 50 to 100 million years between each organ evolving, and it being fed upon.[9] Hole feeding and skeletonization are recorded in the early Permian, with surface fluid feeding evolving by the end of that period.[7]
Co-evolution
Herbivores are dependent on plants for food, and have evolved mechanisms to obtain this food despite the evolution of a diverse arsenal of plant defenses. Herbivore adaptations to plant defense have been likened to offensive traits and consist of adaptations that allow increased feeding and use of a host plant.[10] Relationships between herbivores and their host plants often results in reciprocal evolutionary change, called co-evolution. When an herbivore eats a plant it selects for plants that can mount a defensive response. In cases where this relationship demonstrates specificity (the evolution of each trait is due to the other), and reciprocity (both traits must evolve), the species are thought to have co-evolved.[11]
The "escape and radiation" mechanism for co-evolution presents the idea that adaptations in herbivores and their host plants have been the driving force behind speciation,[4][12] and have played a role in the radiation of insect species during the age of angiosperms.[13] Some herbivores have evolved ways to hijack plant defenses to their own benefit, by sequestering these chemicals and using them to protect themselves from predators.[4] Plant defenses against herbivores are generally not complete so plants also tend to evolve some tolerance to herbivory.
Types
Plant defenses can be classified generally as constitutive or induced. Constitutive defenses are always present in the plant, while induced defenses are produced or mobilized to the site where a plant is injured. There is wide variation in the composition and concentration of constitutive defenses and these range from mechanical defenses to digestibility reducers and toxins. Many external mechanical defenses and large quantitative defenses are constitutive, as they require large amounts of resources to produce and are difficult to mobilize.[14] A variety of molecular and biochemical approaches are used to determine the mechanism of constitutive and induced plant defenses responses against herbivory.[15][16][17][18]
Induced defenses include secondary metabolic products, as well as morphological and physiological changes.[19] An advantage of inducible, as opposed to constitutive defenses, is that they are only produced when needed, and are therefore potentially less costly, especially when herbivory is variable.[19] Modes of induced defence include systemic acquired resistance[20] and plant-induced systemic resistance.[21]
Chemical defenses

The evolution of chemical defenses in plants is linked to the emergence of chemical substances that are not involved in the essential photosynthetic and metabolic activities. These substances, secondary metabolites, are organic compounds that are not directly involved in the normal growth, development or reproduction of organisms,[22] and often produced as by-products during the synthesis of primary metabolic products.[23] Although these secondary metabolites have been thought to play a major role in defenses against herbivores,[4][22][24] a meta-analysis of recent relevant studies has suggested that they have either a more minimal (when compared to other non-secondary metabolites, such as primary chemistry and physiology) or more complex involvement in defense.[25]
Qualitative and quantitative metabolites
Secondary metabolites are often characterized as either qualitative or quantitative. Qualitative metabolites are defined as toxins that interfere with a herbivore's metabolism, often by blocking specific biochemical reactions. Qualitative chemicals are present in plants in relatively low concentrations (often less than 2% dry weight), and are not dosage dependent. They are usually small, water-soluble molecules, and therefore can be rapidly synthesized, transported and stored with relatively little energy cost to the plant. Qualitative allelochemicals are usually effective against non-adapted generalist herbivores.
Quantitative chemicals are those that are present in high concentration in plants (5 – 40% dry weight) and are equally effective against all specialists and generalist herbivores. Most quantitative metabolites are digestibility reducers that make plant cell walls indigestible to animals. The effects of quantitative metabolites are dosage dependent and the higher these chemicals’ proportion in the herbivore’s diet, the less nutrition the herbivore can gain from ingesting plant tissues. Because they are typically large molecules, these defenses are energetically expensive to produce and maintain, and often take longer to synthesize and transport.[26]
The geranium, for example, produces a unique chemical compound in its petals to defend itself from Japanese beetles. Within 30 minutes of ingestion the chemical paralyzes the herbivore. While the chemical usually wears off within a few hours, during this time the beetle is often consumed by its own predators.[27]
Antiherbivory compounds
Plants have evolved many secondary metabolites involved in plant defense, which are collectively known as antiherbivory compounds and can be classified into three sub-groups: nitrogen compounds (including alkaloids, cyanogenic glycosides, glucosinolates and benzoxazinoids), terpenoids, and phenolics.[28]
Alkaloids are derived from various amino acids. Over 3000 known alkaloids exist, examples include nicotine, caffeine, morphine, cocaine, colchicine, ergolines, strychnine, and quinine.[29] Alkaloids have pharmacological effects on humans and other animals. Some alkaloids can inhibit or activate enzymes, or alter carbohydrate and fat storage by inhibiting the formation phosphodiester bonds involved in their breakdown.[30] Certain alkaloids bind to nucleic acids and can inhibit synthesis of proteins and affect DNA repair mechanisms. Alkaloids can also affect cell membrane and cytoskeletal structure causing the cells to weaken, collapse, or leak, and can affect nerve transmission.[31] Although alkaloids act on a diversity of metabolic systems in humans and other animals, they almost uniformly invoke an aversively bitter taste.[32]
Cyanogenic glycosides are stored in inactive forms in plant vacuoles. They become toxic when herbivores eat the plant and break cell membranes allowing the glycosides to come into contact with enzymes in the cytoplasm releasing hydrogen cyanide which blocks cellular respiration.[33] Glucosinolates are activated in much the same way as cyanogenic glucosides, and the products can cause gastroenteritis, salivation, diarrhea, and irritation of the mouth.[32] Benzoxazinoids, secondary defence metabolites, which are characteristic for grasses (Poaceae), are also stored as inactive glucosides in the plant vacuole.[34] Upon tissue disruption they get into contact with β-glucosidases from the chloroplasts, which enzymatically release the toxic aglucones. Whereas some benzoxazinoids are constitutively present, others are only synthesised following herbivore infestation, and thus, considered inducible plant defenses against herbivory.[35]
The terpenoids, sometimes referred to as isoprenoids, are organic chemicals similar to terpenes, derived from five-carbon isoprene units. There are over 10,000 known types of terpenoids.[36] Most are multicyclic structures which differ from one another in both functional groups, and in basic carbon skeletons.[37] Monoterpenoids, continuing 2 isoprene units, are volatile essential oils such as citronella, limonene, menthol, camphor, and pinene. Diterpenoids, 4 isoprene units, are widely distributed in latex and resins, and can be quite toxic. Diterpenes are responsible for making Rhododendron leaves poisonous. Plant steroids and sterols are also produced from terpenoid precursors, including vitamin D, glycosides (such as digitalis) and saponins (which lyse red blood cells of herbivores).[38]
Phenolics, sometimes called phenols, consist of an aromatic 6-carbon ring bonded to a hydroxy group. Some phenols have antiseptic properties, while others disrupt endocrine activity. Phenolics range from simple tannins to the more complex flavonoids that give plants much of their red, blue, yellow, and white pigments. Complex phenolics called polyphenols are capable of producing many different types of effects on humans, including antioxidant properties. Some examples of phenolics used for defense in plants are: lignin, silymarin and cannabinoids.[39] Condensed tannins, polymers composed of 2 to 50 (or more) flavonoid molecules, inhibit herbivore digestion by binding to consumed plant proteins and making them more difficult for animals to digest, and by interfering with protein absorption and digestive enzymes.[40]
In addition, some plants use fatty acid derivates, amino acids and even peptides[41] as defenses. The cholinergic toxine, cicutoxin of water hemlock, is a polyyne derived from the fatty acid metabolism.[42] β-N-Oxalyl-L-α,β-diaminopropionic acid as simple amino acid is used by the sweet pea which leads also to intoxication in humans.[43] The synthesis of fluoroacetate in several plants is an example of the use of small molecules to disrupt the metabolism of herbivores, in this case the citric acid cycle.[44]
In tropical Sargassum and Turbinaria species that are often preferentially consumed by herbivorous fishes and echinoids, there is a relatively low level of phenolics and tannins.[45]
Mechanical defenses

Many plants have external structural defenses that discourage herbivory. Structural defenses can be described as morphological or physical traits that give the plant a fitness advantage by deterring herbivores from feeding.[46] Depending on the herbivore's physical characteristics (i.e. size and defensive armor), plant structural defenses on stems and leaves can deter, injure, or kill the grazer. Some defensive compounds are produced internally but are released onto the plant's surface; for example, resins, lignins, silica, and wax cover the epidermis of terrestrial plants and alter the texture of the plant tissue. The leaves of holly plants, for instance, are very smooth and slippery making feeding difficult. Some plants produce gummosis or sap that traps insects.[47]
Spines and thorns
A plant's leaves and stem may be covered with sharp prickles, spines, thorns, or trichomes- hairs on the leaf often with barbs, sometimes containing irritants or poisons. Plant structural features like spines and thorns reduce feeding by large ungulate herbivores (e.g. kudu, impala, and goats) by restricting the herbivores' feeding rate, or by wearing down the molars.[48] Trichomes are frequently associated with lower rates of plant tissue digestion by insect herbivores.[46] Raphides are sharp needles of calcium oxalate or calcium carbonate in plant tissues, making ingestion painful, damaging a herbivore's mouth and gullet and causing more efficient delivery of the plant's toxins. The structure of a plant, its branching and leaf arrangement may also be evolved to reduce herbivore impact. The shrubs of New Zealand have evolved special wide branching adaptations believed to be a response to browsing birds such as the moas.[49] Similarly, African Acacias have long spines low in the canopy, but very short spines high in the canopy, which is comparatively safe from herbivores such as giraffes.[50][51]

Trees such as palms protect their fruit by multiple layers of armor, needing efficient tools to break through to the seed contents. Some plants, notably the grasses, use indigestible silica (and many plants use other relatively indigestible materials such as lignin) to defend themselves against vertebrate and invertebrate herbivores.[52] Plants take up silicon from the soil and deposit it in their tissues in the form of solid silica phytoliths. These mechanically reduce the digestibility of plant tissue, causing rapid wear to vertebrate teeth and insect mandibles,[53] and are effective against herbivores above and below ground.[54] The mechanism may offer future sustainable pest control strategies.[55]
Thigmonastic movements
Thigmonastic movements, those that occur in response to touch, are used as a defense in some plants. The leaves of the sensitive plant, Mimosa pudica, close up rapidly in response to direct touch, vibration, or even electrical and thermal stimuli. The proximate cause of this mechanical response is an abrupt change in the turgor pressure in the pulvini at the base of leaves resulting from osmotic phenomena. This is then spread via both electrical and chemical means through the plant; only a single leaflet need be disturbed. This response lowers the surface area available to herbivores, which are presented with the underside of each leaflet, and results in a wilted appearance. It may also physically dislodge small herbivores, such as insects.[56]
Mimicry and camouflage
Some plants mimic the presence of insect eggs on their leaves, dissuading insect species from laying their eggs there. Because female butterflies are less likely to lay their eggs on plants that already have butterfly eggs, some species of neotropical vines of the genus Passiflora (Passion flowers) contain physical structures resembling the yellow eggs of Heliconius butterflies on their leaves, which discourage oviposition by butterflies.[57]
Indirect defenses

Another category of plant defenses are those features that indirectly protect the plant by enhancing the probability of attracting the natural enemies of herbivores. Such an arrangement is known as mutualism, in this case of the "enemy of my enemy" variety. One such feature are semiochemicals, given off by plants. Semiochemicals are a group of volatile organic compounds involved in interactions between organisms. One group of semiochemicals are allelochemicals; consisting of allomones, which play a defensive role in interspecies communication, and kairomones, which are used by members of higher trophic levels to locate food sources. When a plant is attacked it releases allelochemics containing an abnormal ratio of these herbivore-induced plant volatiles (HIPVs).[58][59] Predators sense these volatiles as food cues, attracting them to the damaged plant, and to feeding herbivores. The subsequent reduction in the number of herbivores confers a fitness benefit to the plant and demonstrates the indirect defensive capabilities of semiochemicals.[60] Induced volatiles also have drawbacks, however; some studies have suggested that these volatiles attract herbivores.[58]
Plants sometimes provide housing and food items for natural enemies of herbivores, known as "biotic" defense mechanisms, as a means to maintain their presence. For example, trees from the genus Macaranga have adapted their thin stem walls to create ideal housing for an ant species (genus Crematogaster), which, in turn, protects the plant from herbivores.[61] In addition to providing housing, the plant also provides the ant with its exclusive food source; from the food bodies produced by the plant. Similarly, several Acacia tree species have developed stipular spines (direct defenses) that are swollen at the base, forming a hollow structure that provides housing for protective ants. These Acacia trees also produce nectar in extrafloral nectaries on their leaves as food for the ants.[62]
Plant use of endophytic fungi in defense is common. Most plants have endophytes, microbial organisms that live within them. While some cause disease, others protect plants from herbivores and pathogenic microbes. Endophytes can help the plant by producing toxins harmful to other organisms that would attack the plant, such as alkaloid producing fungi which are common in grasses such as tall fescue (Festuca arundinacea).[56]
Leaf shedding and color
There have been suggestions that leaf shedding may be a response that provides protection against diseases and certain kinds of pests such as leaf miners and gall forming insects.[63] Other responses such as the change of leaf colors prior to fall have also been suggested as adaptations that may help undermine the camouflage of herbivores.[64] Autumn leaf color has also been suggested to act as an honest warning signal of defensive commitment towards insect pests that migrate to the trees in autumn.[65][66]
Costs and benefits
Defensive structures and chemicals are costly as they require resources that could otherwise be used by plants to maximize growth and reproduction. Many models have been proposed to explore how and why some plants make this investment in defenses against herbivores.
Optimal defense hypothesis
The optimal defense hypothesis attempts to explain how the kinds of defenses a particular plant might use reflect the threats each individual plant faces.[67] This model considers three main factors, namely: risk of attack, value of the plant part, and the cost of defense.[68][69]
The first factor determining optimal defense is risk: how likely is it that a plant or certain plant parts will be attacked? This is also related to the plant apparency hypothesis, which states that a plant will invest heavily in broadly effective defenses when the plant is easily found by herbivores.[70] Examples of apparent plants that produce generalized protections include long-living trees, shrubs, and perennial grasses.[70] Unapparent plants, such as short-lived plants of early successional stages, on the other hand, preferentially invest in small amounts of qualitative toxins that are effective against all but the most specialized herbivores.[70]
The second factor is the value of protection: would the plant be less able to survive and reproduce after removal of part of its structure by a herbivore? Not all plant parts are of equal evolutionary value, thus valuable parts contain more defenses. A plant’s stage of development at the time of feeding also affects the resulting change in fitness. Experimentally, the fitness value of a plant structure is determined by removing that part of the plant and observing the effect.[71] In general, reproductive parts are not as easily replaced as vegetative parts, terminal leaves have greater value than basal leaves, and the loss of plant parts mid-season has a greater negative effect on fitness than removal at the beginning or end of the season.[72][73] Seeds in particular tend to be very well protected. For example, the seeds of many edible fruits and nuts contain cyanogenic glycosides such as amygdalin. This results from the need to balance the effort needed to make the fruit attractive to animal dispersers while ensuring that the seeds are not destroyed by the animal.[74][75]
The final consideration is cost: how much will a particular defensive strategy cost a plant in energy and materials? This is particularly important, as energy spent on defense cannot be used for other functions, such as reproduction and growth. The optimal defense hypothesis predicts that plants will allocate more energy towards defense when the benefits of protection outweigh the costs, specifically in situations where there is high herbivore pressure.[76]
Carbon:nutrient balance hypothesis
The carbon:nutrient balance hypothesis, also known as the environmental constraint hypothesis or Carbon Nutrient Balance Model (CNBM), states that the various types of plant defenses are responses to variations in the levels of nutrients in the environment.[77][78] This hypothesis predicts the Carbon/Nitrogen ratio in plants determines which secondary metabolites will be synthesized. For example, plants growing in nitrogen-poor soils will use carbon-based defenses (mostly digestibility reducers), while those growing in low-carbon environments (such as shady conditions) are more likely to produce nitrogen-based toxins. The hypothesis further predicts that plants can change their defenses in response to changes in nutrients. For example, if plants are grown in low-nitrogen conditions, then these plants will implement a defensive strategy composed of constitutive carbon-based defenses. If nutrient levels subsequently increase, by for example the addition of fertilizers, these carbon-based defenses will decrease.
Growth rate hypothesis
The growth rate hypothesis, also known as the resource availability hypothesis, states that defense strategies are determined by the inherent growth rate of the plant, which is in turn determined by the resources available to the plant. A major assumption is that available resources are the limiting factor in determining the maximum growth rate of a plant species. This model predicts that the level of defense investment will increase as the potential of growth decreases.[79] Additionally, plants in resource-poor areas, with inherently slow-growth rates, tend to have long-lived leaves and twigs, and the loss of plant appendages may result in a loss of scarce and valuable nutrients.[80]
A recent test of this model involved a reciprocal transplants of seedlings of 20 species of trees between clay soils (nutrient rich) and white sand (nutrient poor) to determine whether trade-offs between growth rate and defenses restrict species to one habitat. When planted in white sand and protected from herbivores, seedlings originating from clay outgrew those originating from the nutrient-poor sand, but in the presence of herbivores the seedlings originating from white sand performed better, likely due to their higher levels of constitutive carbon-based defenses. These finding suggest that defensive strategies limit the habitats of some plants.[81]
Growth-differentiation balance hypothesis
The growth-differentiation balance hypothesis states that plant defenses are a result of a tradeoff between "growth-related processes" and "differentiation-related processes" in different environments.[82] Differentiation-related processes are defined as "processes that enhance the structure or function of existing cells (i.e. maturation and specialization)."[67] A plant will produce chemical defenses only when energy is available from photosynthesis, and plants with the highest concentrations of secondary metabolites are the ones with an intermediate level of available resources.[82]
The GDBH also accounts for tradeoffs between growth and defense over a resource availability gradient. In situations where resources (e.g. water and nutrients) limit photosynthesis, carbon supply is predicted to limit both growth and defense. As resource availability increases, the requirements needed to support photosynthesis are met, allowing for accumulation of carbohydrate in tissues. As resources are not sufficient to meet the large demands of growth, these carbon compounds can instead be partitioned into the synthesis of carbon based secondary metabolites (phenolics, tannins, etc.). In environments where the resource demands for growth are met, carbon is allocated to rapidly dividing meristems (high sink strength) at the expense of secondary metabolism. Thus rapidly growing plants are predicted to contain lower levels of secondary metabolites and vice versa. In addition, the tradeoff predicted by the GDBH may change over time, as evidenced by a recent study on Salix spp. Much support for this hypothesis is present in the literature, and some scientists consider the GDBH the most mature of the plant defense hypotheses.
Importance to humans
Agriculture
The variation of plant susceptibility to pests was probably known even in the early stages of agriculture in humans. In historic times, the observation of such variations in susceptibility have provided solutions for major socio-economic problems. The hemipteran pest insect phylloxera was introduced from North America to France in 1860 and in 25 years it destroyed nearly a third (100,000 km²) of French vineyards. Charles Valentine Riley noted that the American species Vitis labrusca was resistant to Phylloxera. Riley, with J. E. Planchon, helped save the French wine industry by suggesting the grafting of the susceptible but high quality grapes onto Vitis labrusca root stocks.[83] The formal study of plant resistance to herbivory was first covered extensively in 1951 by Reginald Henry Painter, who is widely regarded as the founder of this area of research, in his book Plant Resistance to Insects.[84] While this work pioneered further research in the US, the work of Chesnokov was the basis of further research in the USSR.[85]
Fresh growth of grass is sometimes high in prussic acid content and can cause poisoning of grazing livestock. The production of cyanogenic chemicals in grasses is primarily a defense against herbivores.[86][87]
The human innovation of cooking may have been particularly helpful in overcoming many of the defensive chemicals of plants. Many enzyme inhibitors in cereal grains and pulses, such as trypsin inhibitors prevalent in pulse crops, are denatured by cooking, making them digestible.[88][89]
It has been known since the late 17th century that plants contain noxious chemicals which are avoided by insects. These chemicals have been used by man as early insecticides; in 1690 nicotine was extracted from tobacco and used as a contact insecticide. In 1773, insect infested plants were treated with nicotine fumigation by heating tobacco and blowing the smoke over the plants.[90] The flowers of Chrysanthemum species contain pyrethrin which is a potent insecticide. In later years, the applications of plant resistance became an important area of research in agriculture and plant breeding, particularly because they can serve as a safe and low-cost alternative to the use of pesticides.[91] The important role of secondary plant substances in plant defense was described in the late 1950s by Vincent Dethier and G.S. Fraenkel.[22][92] The use of botanical pesticides is widespread and notable examples include Azadirachtin from the neem (Azadirachta indica), d-Limonene from Citrus species, Rotenone from Derris, Capsaicin from chili pepper and Pyrethrum.[93]
Natural materials found in the environment also induce plant resistance as well.[94] Chitosan derived from chitin induce a plant's natural defense response against pathogens, diseases and insects including cyst nematodes, both are approved as biopesticides by the EPA to reduce the dependence on toxic pesticides.
The selective breeding of crop plants often involves selection against the plant's intrinsic resistance strategies. This makes crop plant varieties particularly susceptible to pests unlike their wild relatives. In breeding for host-plant resistance, it is often the wild relatives that provide the source of resistance genes. These genes are incorporated using conventional approaches to plant breeding, but have also been augmented by recombinant techniques, which allow introduction of genes from completely unrelated organisms. The most famous transgenic approach is the introduction of genes from the bacterial species, Bacillus thuringiensis, into plants. The bacterium produces proteins that, when ingested, kill lepidopteran caterpillars. The gene encoding for these highly toxic proteins, when introduced into the host plant genome, confers resistance against caterpillars, when the same toxic proteins are produced within the plant. This approach is controversial, however, due to the possibility of ecological and toxicological side effects.[95]
Pharmaceutical

Many currently available pharmaceuticals are derived from the secondary metabolites plants use to protect themselves from herbivores, including opium, aspirin, cocaine, and atropine.[96] These chemicals have evolved to affect the biochemistry of insects in very specific ways. However, many of these biochemical pathways are conserved in vertebrates, including humans, and the chemicals act on human biochemistry in ways similar to that of insects. It has therefore been suggested that the study of plant-insect interactions may help in bioprospecting.[97]
There is evidence that humans began using plant alkaloids in medical preparations as early as 3000 B.C.[30] Although the active components of most medicinal plants have been isolated only recently (beginning in the early 19th century) these substances have been used as drugs throughout the human history in potions, medicines, teas and as poisons. For example, to combat herbivory by the larvae of some Lepidoptera species, Cinchona trees produce a variety of alkaloids, the most familiar of which is quinine. Quinine is extremely bitter, making the bark of the tree quite unpalatable, it is also an anti-fever agent, known as Jesuit's bark, and is especially useful in treating malaria.[98]
Throughout history mandrakes (Mandragora officinarum) have been highly sought after for their reputed aphrodisiac properties. However, the roots of the mandrake plant also contain large quantities of the alkaloid scopolamine, which, at high doses, acts as a central nervous system depressant, and makes the plant highly toxic to herbivores. Scopolamine was later found to be medicinally used for pain management prior to and during labor; in smaller doses it is used to prevent motion sickness.[99] One of the most well-known medicinally valuable terpenes is an anticancer drug, taxol, isolated from the bark of the Pacific yew, Taxus brevifolia, in the early 1960s.[100]
Biological pest control
Repellent companion planting, defensive live fencing hedges, and "obstructive-repellent" interplanting, with host-plant resistance species as beneficial 'biological control agents' is a technique in biological pest control programs for: organic gardening, wildlife gardening, sustainable gardening, and sustainable landscaping; in organic farming and sustainable agriculture; and in restoration ecology methods for habitat restoration projects.
See also
- Anti-predator adaptation
- Aposematism
- Biopesticide
- Chemical ecology
- Canavanine
- Druse (botany)
- Laticifer
- Lectin
- List of beneficial weeds
- List of companion plants
- List of pest-repelling plants
- Plant disease resistance
- Plant tolerance to herbivory
- Pollination
- Phytoalexin
- Raphide
- Rapid plant movement
- Seed predation
- Tritrophic interactions in plant defense
References
Citations
- Boyd, Jade (2012). "A bit touchy: Plants' insect defenses activated by touch". Rice University. http://news.rice.edu/2012/04/09/a-bit-touchy-plants-insect-defenses-activated-by-touch-2/
- Venturi, S.; Donati, F.M.; Venturi, A.; Venturi, M. (2000). "Environmental Iodine Deficiency: A Challenge to the Evolution of Terrestrial Life?". Thyroid. 10 (8): 727–9. doi:10.1089/10507250050137851. PMID 11014322.
- Venturi, Sebastiano (2011). "Evolutionary Significance of Iodine". Current Chemical Biology. 5 (3): 155–162. doi:10.2174/187231311796765012.
- Ehrlich, Paul R.; Peter H. Raven (December 1964). "Butterflies and plants: a study of coevolution". Evolution. 18 (4): 586–608. doi:10.2307/2406212. JSTOR 2406212.
- Labandeira, C.C.; D.L. Dilcher, D.R. Davis, D.L. Wagner; Davis, D. R.; Wagner, D. L. (1994). "Ninety-seven million years of angiosperm-insect association: paleobiological insights into the meaning of coevolution" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 91 (25): 12278–82. Bibcode:1994PNAS...9112278L. doi:10.1073/pnas.91.25.12278. PMC 45420. PMID 11607501.CS1 maint: multiple names: authors list (link)
- Keddy, P.A. 2007. Plants and Vegetation: Origins, Processes, Consequences. Cambridge University Press, Cambridge, UK. 666 p. Chapter 7.
- Labandeira, C.C. (1998). "Early History Of Arthropod And Vascular Plant Associations 1". Annual Review of Earth and Planetary Sciences. 26 (1): 329–377. Bibcode:1998AREPS..26..329L. doi:10.1146/annurev.earth.26.1.329.
- Howe, Henry F.; Westley, Lynn C. (1988). Ecological Relationships of Plants and Animals. New York: Oxford University Press. pp. 29. ISBN 978-0-19-504431-7.
- Labandeira, C. (2007). "The origin of herbivory on land: Initial patterns of plant tissue consumption by arthropods". Insect Science. 14 (4): 259–275. doi:10.1111/j.1744-7917.2007.00152.x.
- Karban, Richard; Anurag A. Agrawal (November 2002). "Herbivore offense". Annual Review of Ecology and Systematics. 33 (1): 641–664. doi:10.1146/annurev.ecolsys.33.010802.150443.
- Futuyma, Douglas J.; Montgomery Slatkin (1983). Coevolution. Sunderland, Massachusetts: Sinauer Associates. ISBN 978-0-87893-228-3.
- Thompson, J. (1999). "What we know and do not know about coevolution: insect herbivores and plants as a test case.". In H. Olff; V. K. Brown; R. H. Drent (eds.). Herbivores: between plants and predators; the 38th symposium of the British Ecological Society in cooperation with the Netherlands Ecological Society held at the Wageningen Agricultural University, The Netherlands, 1997. Oxford: Blackwell Science. pp. 7–30. ISBN 978-0-632-05155-7.
- Farrell, Brian D.; Charles Mitter (1994). "Adaptive Radiation in Insects and Plants: Time and Opportunity". American Zoologist. 34 (1): 57–69. doi:10.1093/icb/34.1.57.
- Traw, Brian M.; Todd E. Dawson (May 2002). "Differential induction of trichomes by three herbivores of black mustard" (PDF). Oecologia. 131 (4): 526–532. Bibcode:2002Oecol.131..526T. doi:10.1007/s00442-002-0924-6. PMID 28547547. Archived from the original (PDF) on 2007-09-27.
- Walling, L.L. (2000). "The myriad plant responses to herbivores". J. Plant Growth Regul. 19 (2): 195–216. doi:10.1007/s003440000026. PMID 11038228.
- Wu, J.; Baldwin, I.T. (2009). "Herbivory-induced signalling in plants: Perception and action". Plant Cell Environ. 32 (9): 1161–1174. doi:10.1111/j.1365-3040.2009.01943.x. PMID 19183291.
- Sarmento, R.A.; Lemos, F.; Dias, C.R.; Kikuchi, W.T.; Rodrigues, J.C.P.; Pallini, A.; Sabelis, M.W.; Janssen, A. (2011). "A herbivorous mite down-regulates plant defence and produces web to exclude competitors". PLOS ONE. 6 (8): e23757. Bibcode:2011PLoSO...623757S. doi:10.1371/journal.pone.0023757. PMC 3161068. PMID 21887311.
- Sangha, J.S.; Yolanda; Chen, H.; Kaur, Jatinder; Khan, Wajahatullah; Abduljaleel, Zainularifeen; Alanazi, Mohammed S.; Mills, Aaron; Adalla, Candida B.; Bennett, John; Prithiviraj, Balakrishnan; Jahn, Gary C.; Leung, Hei (2013). "Proteome Analysis of Rice (Oryza sativa L.) Mutants Reveals Differentially Induced Proteins during Brown Planthopper (Nilaparvata lugens) Infestation". International Journal of Molecular Sciences. 14 (2): 3921–3945. doi:10.3390/ijms14023921. PMC 3588078. PMID 23434671.
- Karban, Richard; Anurag A. Agrawal; Marc Mangel (July 1997). "The benefits of induced defenses against herbivores". Ecology. 78 (5): 1351–1355. doi:10.2307/2266130. hdl:1813/66776. JSTOR 2266130. Retrieved 2007-05-27.
- Conrath, Uwe (2006). "Systemic Acquired Resistance". Plant Signaling & Behavior. 1 (4): 179–184. doi:10.4161/psb.1.4.3221. PMC 2634024. PMID 19521483.
- Choudhary, Devendra K.; Prakash, Anil; Johri, B. N. (December 2007). "Induced systemic resistance (ISR) in plants: mechanism of action". Indian Journal of Microbiology. 47 (4): 289–297. doi:10.1007/s12088-007-0054-2. PMC 3450033. PMID 23100680.
- Fraenkel, G. (1959). "The raison d'être of secondary plant substances". Science. 129 (3361): 1466–70. Bibcode:1959Sci...129.1466F. doi:10.1126/science.129.3361.1466. PMID 13658975.
- Whittaker, Robert H. (1970). "The biochemical ecology of higher plants". In Ernest Sondheimer; John B. Simeone (eds.). Chemical ecology. Boston: Academic Press. pp. 43–70. ISBN 978-0-12-654750-4.
- Whittaker, Robert H. (1975). Communities and ecosystems. New York: Macmillan. ISBN 978-0-02-427390-1.
- Carmona, Diego; Marc J. Lajeunesse; Marc T.J. Johnson (April 2011). "Plant traits that predict resistance to herbivores" (PDF). Functional Ecology. 25 (2): 358–367. doi:10.1111/j.1365-2435.2010.01794.x. Retrieved 26 June 2011.
- Theis, Nina; Manuel Lerdau (2003). "The evolution of function in plant secondary metabolites" (PDF). International Journal of Plant Sciences. 164 (3 Suppl): S93–S102. doi:10.1086/374190. Archived from the original (PDF) on 2007-04-18.
- Flores, Alfredo (March 2010). "Geraniums and Begonias: New Research on Old Garden Favorites". AgResearch Magazine. United States Department of Agriculture.
- "Biochemical defenses: secondary metabolites". Plant Defense Systems & Medicinal Botany. Retrieved 2007-05-21.
- "Alkaloids: contain a N-containing heterocycle". Plant Defense Systems & Medicinal Botany. Retrieved 2007-06-26.
- Roberts, Margaret F.; Michael Wink (1998). Alkaloids: biochemistry, ecology, and medicinal applications. New York: Plenum Press. ISBN 978-0-306-45465-3.
- Sneden, Albert T. "Alkaloids". Natural Products as Medicinally Useful Agents. Archived from the original on 2007-06-02. Retrieved 2007-05-21.
- Rhoades, David F (1979). "Evolution of Plant Chemical Defense against Herbivores". In Rosenthal, Gerald A.; Janzen, Daniel H. (eds.). Herbivores: Their Interaction with Secondary Plant Metabolites. New York: Academic Press. pp. 3–54. ISBN 978-0-12-597180-5.
- Toxicon Volume 38, Issue 1, January 2000, Pages 11-36 János Vetter Plant cyanogenic glycosides doi:10.1016/S0041-0101(99)00128-2
- Niemeyer, HM (2009). "Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: key defense chemicals of cereals". J Agric Food Chem. 57 (5): 1677–1696. doi:10.1021/jf8034034. PMID 19199602.
- Glauser, G; Marti, G; Villard, N; Doyen, GA; Wolfender, J-L; Turlings, TCJ; Erb, M (2011). "Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores". Plant Journal. 68 (5): 901–911. doi:10.1111/j.1365-313X.2011.04740.x. PMID 21838747.
- "Terpenoids". Plant Defense Systems & Medicinal Botany. Retrieved 2007-06-26.
- Gershenzon, Jonathan; Wolfgang Kreis (1999). "Biochemistry of terpenoids". In Michael Wink (ed.). Biochemistry of plant secondary metabolism. London: Sheffield Academic Press. pp. 222–279. ISBN 978-0-8493-4085-7.
- Sneden, Albert T. "Terpenes". Natural Products as Medicinally Useful Agents. Archived from the original on 2007-07-16. Retrieved 2007-05-21.
- "Phenols". Plant Defense Systems & Medicinal Botany. Retrieved 2007-05-21.
- Van Soest, Peter J. (1982). Nutritional ecology of the ruminant: ruminant metabolism, nutritional strategies, the cellulolytic fermentation, and the chemistry of forages and plant fibers. Corvallis, Oregon: O & B Books. ISBN 978-0-9601586-0-7.
- John W. Hylin (1969). "Toxic peptides and amino acids in foods and feeds". Journal of Agricultural and Food Chemistry. 17 (3): 492–496. doi:10.1021/jf60163a003.
- E. Anet; B. Lythgoe; M. H. Silk; S. Trippett (1953). "Oenanthotoxin and cicutoxin. Isolation and structures". Journal of the Chemical Society: 309–322. doi:10.1039/JR9530000309.
- Mark V. Barrow; Charles F. Simpson; Edward J. Miller (1974). "Lathyrism: A Review". The Quarterly Review of Biology. 49 (2): 101–128. doi:10.1086/408017. JSTOR 2820941. PMID 4601279.
- Donald A. Levin; King, Dennis R. (1991). "The Impact of Fluoroacetate-Bearing Vegetation on Native Australian Fauna: A Review". Oikos. 61 (3): 412–430. doi:10.2307/3545249. JSTOR 3545249.
- Steinberg, Peter D. (1986). "Chemical defenses and the susceptibility of tropical marine brown algae to herbivores". Oecologia. 69 (4): 628–630. Bibcode:1986Oecol..69..628S. doi:10.1007/BF00410374. PMID 28311627.
- Hanley, Mick E.; Lamont, Byron B.; Fairbanks, Meredith M.; Rafferty, Christine M. (2007). "Plant structural traits and their role in anti-herbivore defence". Perspectives in Plant Ecology, Evolution and Systematics. 8 (4): 157–178. doi:10.1016/j.ppees.2007.01.001.
- Fernandes, G. W. (1994). "Plant mechanical defenses against insect herbivory". Revista Brasileira de Entomologia. 38 (2): 421–433 .
- Cooper, Susan M.; Owen-Smith, Norman (September 1986). "Effects of plant spinescence on large mammalian herbivores". Oecologia. 68 (3): 446–455. Bibcode:1986Oecol..68..446C. doi:10.1007/BF01036753. PMID 28311793.
- Bond, W.; Lee, W.; Craine, J. (2004). "Plant structural defences against browsing birds: a legacy of New Zealand's extinct moas". Oikos. 104 (3): 500–508. doi:10.1111/j.0030-1299.2004.12720.x.
- Young, Truman P. (1987). "Increased thorn length in Acacia drepanolobium- an induced response to browsing". Oecologia. 71 (3): 436–438. Bibcode:1987Oecol..71..436Y. CiteSeerX 10.1.1.536.5315. doi:10.1007/BF00378718. PMID 28312992.
- Young, Truman P.; Bell Okello (1998). "Relaxation of an induced defense after exclusion of herbivores: spines on Acacia drepanolobium". Oecologia. 115 (4): 508–513. Bibcode:1998Oecol.115..508Y. doi:10.1007/s004420050548. PMID 28308271.
- Epstein, E. (2009). "Silicon: its manifold roles in plants". Annals of Applied Biology. 155 (2): 155–160. doi:10.1111/j.1744-7348.2009.00343.x.
- Massey F. P.; Hartley S. E. (2009). "Physical defences wear you down: progressive and irreversible impacts of silica on insect herbivores". Journal of Animal Ecology. 78 (1): 281–291. doi:10.1111/j.1365-2656.2008.01472.x. PMID 18771503.
- Frew, A.; Powell, J. R.; Sallam, N.; Allsopp, P. G.; Johnson, S. N. (2016). "Trade-offs between silicon and phenolic defenses may explain enhanced performance of root herbivores on phenolic-rich plants". Journal of Chemical Ecology. 42 (8): 768–771. doi:10.1007/s10886-016-0734-7. PMID 27481346.
- Frew, A.; Allsopp, P. G.; Gherlenda, A. G.; Johnson, S. N. (2016). "Increased root herbivory under elevated atmospheric carbon dioxide concentrations is reversed by silicon-based plant defences". Journal of Applied Ecology. 54 (5): 1310–1319. doi:10.1111/1365-2664.12822.
- Raven, Peter H.; Ray F. Evert; Susan E. Eichhorn (2005). Biology of Plants. New York: W. H. Freeman and Company. ISBN 978-0-7167-1007-3.
- Williams, Kathy S.; Lawrence E. Gilbert (April 1981). "Insects as selective agents on plant vegetative morphology: egg mimicry reduces egg-laying by butterflies". Science. 212 (4493): 467–469. Bibcode:1981Sci...212..467W. doi:10.1126/science.212.4493.467. PMID 17802547.
- Dicke, Marcel; Joop J.A. van Loon (December 2000). "Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context". Entomologia Experimentalis et Applicata. 97 (3): 237–249. doi:10.1046/j.1570-7458.2000.00736.x.
- Allmann, S.; Baldwin, I. T. (2010). "Insects Betray Themselves in Nature to Predators by Rapid Isomerization of Green Leaf Volatiles". Science. 329 (5995): 1075–8. Bibcode:2010Sci...329.1075A. doi:10.1126/science.1191634. PMID 20798319.
- Schuman, Meredith C.; Barthel, Kathleen; Baldwin, Ian T. (October 2012). "Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature". eLife. 1: e00007. doi:10.7554/eLife.00007. PMC 3466783. PMID 23066503.
- Heil, Martin; Brigitte Fiala, K. Eduard Linsenmair, Gerhard Zotz, Petra Menke (December 1997). "Food body production in Macaranga triloba (Euphorbiaceae): A plant investment in anti-herbivore defense via symbiotic ant partners". Journal of Ecology. 85 (6): 847–861. doi:10.2307/2960606. JSTOR 2960606.CS1 maint: multiple names: authors list (link)
- Young, Truman P.; Cynthia H. Stubblefield; Lynne A. Isbell (January 1997). "Ants on swollen-thorn acacias: species coexistence in a simple system". Oecologia. 109 (1): 98–107. Bibcode:1997Oecol.109...98Y. doi:10.1007/s004420050063. PMID 28307618.
- Williams, Alan G.; Thomas G. Whitham (December 1986). "Premature Leaf Abscission: An Induced Plant Defense Against Gall Aphids". Ecology. 67 (6): 1619–1627. doi:10.2307/1939093. JSTOR 1939093.
- Lev-Yadun, Simcha; Amots Dafni; Moshe A. Flaishman; Moshe Inbar; Ido Izhaki; Gadi Katzir; Gidi Ne'eman (October 2004). "Plant coloration undermines herbivorous insect camouflage" (PDF). BioEssays. 26 (10): 1126–1130. doi:10.1002/bies.20112. PMID 15382135. Archived from the original (PDF) on 2007-11-27. Retrieved 2007-05-27.
- Archetti, M. (2000). "The origin of autumn colours by coevolution". J. Theor. Biol. 205 (4): 625–630. doi:10.1006/jtbi.2000.2089. PMID 10931756.
- Hamilton, W. D.; Brown, S. P. (2001). "Autumn tree colours as a handicap signal". Proc. R. Soc. B. 268 (1475): 1489–1493. doi:10.1098/rspb.2001.1672. PMC 1088768. PMID 11454293.
- Stamp, Nancy (March 2003). "Out of the quagmire of plant defense hypotheses". Quarterly Review of Biology. 78 (1): 23–55. doi:10.1086/367580. PMID 12661508.
- Rhoades, D. F.; R. G. Cates. (1974). "Towards a general theory of plant antiherbivore chemistry". In V. C. Runeckles; E. E. Conn (eds.). Recent advances in phytochemistry: proceedings of the annual meeting of the Phytochemical society of North America. Boston: Academic Press. pp. 168–213. ISBN 978-0-12-612408-8.
- Wilf, Peter; Conrad C. Labandeira; Kirk R. Johnson; Phyllis D. Coley; Asher D. Cutter (2001). "Insect herbivory, plant defense, and early Cenozoic climate change" (PDF). Proceedings of the National Academy of Sciences. 98 (11): 6221–6226. Bibcode:2001PNAS...98.6221W. doi:10.1073/pnas.111069498. PMC 33449. PMID 11353840. Retrieved 2007-05-27.
- Feeny, P. (1976). "Plant apparency and chemical defense.". In James W. Wallace; Richard L. Mansell (eds.). Biochemical interaction between plants and insects: proceedings of the fifteenth annual meeting of the Phytochemical Society of North America. New York: Plenum Press. pp. 1–40. ISBN 978-0-306-34710-8.
- D., McKey (1979). "The distribution of secondary compounds within plants.". In Gerald A. Rosenthal; Daniel H. Janzen (eds.). Herbivores, their interaction with secondary plant metabolites. Boston: Academic Press. pp. 55–133. ISBN 978-0-12-597180-5.
- Krischik, V. A.; R. F. Denno (1983). "Individual, population, and geographic patterns in plant defense.". In Robert F. Denno; Mark S. McClure (eds.). Variable plants and herbivores in natural and managed systems. Boston: Academic Press. pp. 463–512. ISBN 978-0-12-209160-5.
- Zangerl, Arthur R.; Claire E. Rutledge (April 1996). "The probability of attack and patterns of constitutive and induced defense: A test of optimal defense theory". The American Naturalist. 147 (4): 599–608. doi:10.1086/285868. JSTOR 2463237.
- Swain, Elisabeth; Chun Ping Li; Jonathan E. Poulton (1992). "Development of the Potential for Cyanogenesis in Maturing Black Cherry (Prunus serotina Ehrh.) Fruits". Plant Physiology. 98 (4): 1423–1428. doi:10.1104/pp.98.4.1423. PMC 1080367. PMID 16668810.
- Witmer, M.C. (1998). "Ecological and evolutionary implications of energy and protein requirements of avian frugivores eating sugary diets". Physiological Zoology. 71 (6): 599–610. doi:10.1086/516001. PMID 9798248.
- Pennings, Steven C.; Erin L. Siska; Mark D. Bertness (May 2001). "Latitudinal differences in plant palatability in Atlantic coast salt marshes". Ecology. 82 (5): 1344–1359. doi:10.2307/2679994. JSTOR 2679994.
- Bryant, John P.; Stuart Chapin, III; David R. Klein (May 1983). "Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory". Oikos. 40 (3): 357–368. doi:10.2307/3544308. JSTOR 3544308.
- Tuomi, J.; P. Niemela; F. S. Chapin, III; J. P. Bryant; S. Siren. (1988). "Defensive responses of trees in relation to their carbon/nutrient balance.". In William J. Mattson; Jean Levieux; C. Bernard-Dagan (eds.). Mechanisms of woody plant defenses against insects: search for pattern. Springer-Verlag. pp. 57–72. ISBN 978-0-387-96673-1.
- Colley, Phyllis D.; John P. Bryant; F. Stuart Chapin III (1985). "Resource availability and plant antiherbivore defense". Science. 230 (4728): 895–899. Bibcode:1985Sci...230..895C. doi:10.1126/science.230.4728.895. PMID 17739203.
- Chapin, F. Stuart, III (1980). "The Mineral Nutrition of Wild Plants". Annual Review of Ecology and Systematics. 11: 233–260. doi:10.1146/annurev.es.11.110180.001313. JSTOR 2096908.
- Fine, Paul V. A.; Italo Mesones; Phyllis D. Coley (July 2004). "Herbivores promote habitat specialization by trees in Amazonian forests". Science. 305 (5684): 663–5. Bibcode:2004Sci...305..663F. doi:10.1126/science.1098982. PMID 15286371.
- Loomis, W. E. (1981). "Growth and differentiation—an introduction and summary.". In P. F. Wareing; I. D. J. Phillips (eds.). Growth and differentiation in plants. New York: Pergamon Press. pp. 1–17. ISBN 978-0-08-026351-9.
Herms, Daniel A.; William J. Mattson (September 1992). "The dilemma of plants: to grow or defend". Quarterly Review of Biology. 67 (3): 283–335. doi:10.1086/417659. JSTOR 2830650. - Polavarapu, Sridhar (2001). "Plant Resistance to insects". Agricultural Entomology & Pest Management. Rutgers University. Archived from the original on 2007-07-13. Retrieved 2007-05-16.
- Painter, Reginald Henry (1951). Insect Resistance in Crop Plants. Lawrence: University of Kansas Press. OCLC 443998.
- Chesnokov, Pavel G. (1953). Methods of Investigating Plant Resistance to Pests. Jerusalem: Israel Program for Scientific Translations. OCLC 3576157.
- Gleadow, Roslyn M.; Ian E. Woodrow (2002). "Constraints on effectiveness of cyanogenic glycosides in herbivore defense". Journal of Chemical Ecology. 28 (7): 1301–13. doi:10.1023/A:1016298100201. PMID 12199497.
- Vough, Lester R.; E. Kim Cassel (July 2002). "Prussic Acid Poisoning of Livestock: Causes and Prevention (ExEx 4016)" (PDF). Extension Extra. South Dakota State University Extension Service. Archived from the original (PDF) on 2007-02-13.
- Grant, G; Linda J. More; Norma H. McKenzie; Arpad Pusztai (1982). "The effect of heating on the haemagglutinating activity and nutritional properties of bean (Phaseolus vulgaris) seeds". Journal of the Science of Food and Agriculture. 33 (12): 1324–6. doi:10.1002/jsfa.2740331220. PMID 7166934.
- Tu Jean-Louis (1999). "Natural Toxins in Raw Foods and How Cooking Affects Them". Is Cooked Food Poison?. Beyond Vegetarianism. Retrieved 2007-05-22.
- George W. (2004). The Pesticide Book. Willoughby: MeisterPro. ISBN 978-1-892829-11-5.
firat Ware
- Michael Smith, C. (2005). Plant Resistance to Arthropods: Molecular and Conventional Approaches. Berlin: Springer. ISBN 978-1-4020-3701-6.
- Dethier, V. G. (March 1954). "Evolution of feeding preferences in phytophagous insects". Evolution. 8 (1): 33–54. doi:10.2307/2405664. JSTOR 2405664.
- Russ, Karen. "Less toxic insecticides" (PDF). Clemson University Home & Garden Information Center. Retrieved 2007-05-27.
- "Linden, J., Stoner, R., Knutson, K. Gardner-Hughes, C. "Organic Disease Control Elicitors". Agro Food Industry Hi-Te (p12-15 Oct 2000)" (PDF). Archived from the original (PDF) on 2007-07-06.
- van Emden, H.F. (November 1999). "Transgenic Host Plant Resistance to Insects—Some Reservations". Annals of the Entomological Society of America. 92 (6): 788–797. doi:10.1093/aesa/92.6.788.
- Ghosh, B. (2000). "Polyamines and plant alkaloids". Indian Journal of Experimental Biology. 38 (11): 1086–91. PMID 11395950.
- Eisner, Thomas (March 1990). "Prospecting for nature's chemical riches". Chemoecology. 1 (1): 38–40. doi:10.1007/BF01240585.
- Albert T. Sneden. "The Quinine Alkaloids" (PDF). Medicinal Chemistry and Drug Design. Archived from the original (PDF) on 2007-02-05. Retrieved 2007-05-23.
- Albert T. Sneden. "The Tropane Alkaloids" (PDF). Medicinal Chemistry and Drug Design. Archived from the original (PDF) on 2007-09-27. Retrieved 2007-05-23.
- Albert T. Sneden. "Taxol (Paclitaxe)" (PDF). Medicinal Chemistry and Drug Design. Archived from the original (PDF) on 2007-09-27. Retrieved 2007-05-23.
Sources
- Robert S. Fritz; Ellen L. Simms, eds. (1992). Plant resistance to herbivores and pathogens: ecology, evolution, and genetics. Chicago: University of Chicago Press. ISBN 978-0-226-26553-7.
- Hartley, Sue (2010) The 300 Million Years War: Plant Biomass v Herbivores Royal Institution Christmas Lecture.
- Howe, H. F., and L. C. Westley. 1988. Ecological relationships of plants and animals. Oxford University Press, Oxford, UK.
- Pierre Jolivet (1998). Interrelationship Between Insects and Plants. Boca Raton: CRC. ISBN 978-1-57444-052-2.
- Richard Karban & Ian T. Baldwin (1997). Induced responses to herbivory. Chicago: University of Chicago Press. ISBN 978-0-226-42495-8.
- Martin R. Speight; Mark D. Hunter; Allan D. Watt (1999). Ecology of insects: concepts and applications. Oxford: Blackwell Science. ISBN 978-0-86542-745-7.
- John N. Thompson (1994). The coevolutionary process. Chicago: University of Chicago Press. ISBN 978-0-226-79759-5.
- Wiens, D. (1978). Mimicry in plants. Evolutionary Biology. 11. pp. 365–403. doi:10.1007/978-1-4615-6956-5_6. ISBN 978-1-4615-6958-9.